ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Internal Thoracic Artery Skeletonization with an Ultrasonic Scalpel
Index
Related Items
Journal
Skeletonization of the internal thoracic artery: a randomized comparison of harvesting methods
Metaanalysis on Skeletonization of the Internal Thoracic Artery
How to Use the Left Internal Thoracic Artery Which Has Been Damaged During Harvesting?
Technique of Harvesting an Internal Thoracic Artery Densely Adherent to the Periosteum
Does a skeletonized or pedicled left internal thoracic artery give the best graft patency?
Should the internal thoracic artery be skeletonized?
Videos
Skeletonization of the Internal Mammary Artery with the Harmonic Hook Blade
Go directly to the video
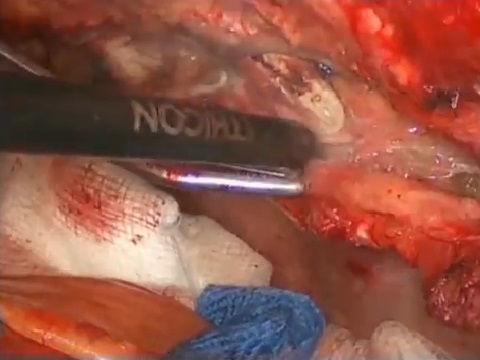 Left internal thoracic artery (ITA) grafting to the left anterior descending artery has long become the gold standard. There is a trend for bilateral ITA grafting, commonly to left-sided arteries. The video shows ITA skeletonization, with low harvesting time, and minimal chest and graft thermal trauma.
Left internal thoracic artery (ITA) grafting to the left anterior descending artery has long become the gold standard. There is a trend for bilateral ITA grafting, commonly to left-sided arteries. The video shows ITA skeletonization, with low harvesting time, and minimal chest and graft thermal trauma.
The use of the left internal thoracic artery (LITA) for revascularization of the left anterior descending (LAD) artery is the gold standard in coronary artery bypass grafting (CABG). When LAD surgical revascularization is indicated, the LITA should be used if possible [1,2], even in revascularization after ST segment elevation myocardial infarction (STEMI) [3] (ACC/AHA Class I, Level of Evidence B, for all recommendations).
Large-scale retrospective studies showed that LITA to LAD grafting was associated with a survival advantage that increased over time, lower incidence of cardiac reoperation, and decreased risk of cardiac events in comparison to vein graft utilization [4,5]
Internal thoracic artery (ITA) use was found to be an independent predictor of survival, decreasing the risk of death in all patient subgroups [4,5]. The excellent long-term patency of ITA grafts, which is superior to that of vein grafts (>/= 90% vs. 40-60% at 10 years) [1,5], is the primary reason for the improved outcomes [6].
Better long-term patency of ITA grafts has led to a trend for extended arterial revascularization, the most common approach of which is bilateral internal thoracic artery (BITA) revascularization. Large-scale, retrospective, nonrandomized comparative studies provided some indication that BITA and vein revascularization were associated with better long-term clinical outcome in comparison to single internal thoracic artery (SITA) and vein revascularization [6-12]. The most common approach in BITA revascularization is arterial grafting of the two most important left-sided coronary arteries [13-15]. The large-scale multicenter randomized arterial revascularization trial (ART) showed similar early mortality and cardiac morbidity in BITA vs. SITA grafting, but BITA grafting was associated with increased incidence of sternal wound reconstruction (1.9% vs. 0.6%), particularly among diabetic patients [15].
According to the current guidelines (ACCF/AHA 2011) when surgical revascularization of the circumflex is indicated the use of a second ITA is reasonable. When surgical revascularization of the right coronary artery (RCA) is indicated the use of a second ITA is considered reasonable, if the RCA is critically stenosed and perfuses the left ventricular myocardium. (Class IIa, Level of evidence B) [2]. BITA grafting in diabetic patients is associated with increased risk of deep sternal wound infection. It may be reasonable if the overall benefit outweighs this risk. (Class IIb, Level of Evidence C). [2]
Preservation of ITA integrity during harvesting is of outmost importance for the early and late graft patency. Minimization of thoracic wall trauma is an important issue particularly in patients at high risk for sternal wound complications. The ITA is harvested by three different techniques—pedicled, semi-skeletonized, and skeletonized—with different effects on these matters. [16-19]. We present ITA skeletonization with an ultrasonic scalpel which is related with decreased arterial thermal trauma and minimal thoracic wall trauma. [19-23]
Description of the Harmonic Scalpel used for skeletonized ITA harvesting
The Harmonic Scalpel (Ethicon, Endo-Surgery, Cincinnati, OH) [20-24] is an ultrasonic dissector coagulator that simultaneously cuts and coagulates tissues. It converts an ultrasonic wave into mechanical energy, vibrating the blade longitudinally over an excursion of 5 to 10 micrometers at a frequency of 55500 Hertz (55.5 Kilohertz). The longitudinal vibration is the scalpel’s primary cutting mechanism enabling the scalpel to incise tissues with high protein density and rich in collagen (such as muscle and fibrous connective tissue). The mechanical energy denatures proteins into a hemostatic coagulum capable of sealing small vessels [20-24]. Apart from tissue incision due to scalpel longitudinal vibration, "cavitational fragmentation" also occurs disrupting low-density tissues (such as fat and parenchyma) and separating tissue planes ahead of the blade tip [21,22,25]. There is no conduction of electricity and thus no interference with the heart rhythm or pacemakers. The generated temperature of about 80° C (50°-100° C) is much lower compared to that generated by electrocautery, which is about 300° C (100°-600° C). The ultrasonic scalpel causes hemostasis with less thermal trauma spread, less eschar formation, less sticking of tissue to the blade, less smoke production, allowing precise dissection with good visibility [20-24].
We use the hand operated Harmonic Synergy Curved Blade (Code: SNGCB) (Ethicon Endo-Surgery) and the UltraCision Harmonic Scalpel Generator 300 System, (Code: GEN04 Ethicon Endo-Surgery, now known as Harmonic Generator 300 System, Code: GEN04, Ethicon Endo-Surgery). The generator supplies the handpiece with electrical energy, which is converted into ultrasonic energy, and finally to mechanical motion. The high-frequency mechanical vibration of the curved blade cuts and simultaneously coagulates tissue. The generator delivers two power levels: a minimum level that can be adjusted from 1 to 5, and a maximum that is always 5. With the Harmonic Synergy Curved Blade scalpel, use of a higher generator power level offers greater tissue cutting speed, while a lower generator power level offers greater coagulation.
In the technique demonstrated in the video, the settings are adjusted at a minimum level of 3 and a maximum level of 5, while most of the harvesting is performed with the high level of energy (level 5).
Operative technique for Curved Blade ultrasonic ITA skeletonization
We have extensively harvested skeletonized ITA grafts with the hand operated Harmonic Synergy Curved Blade using a technique following the principals described by Higami et al. [21-23]. Our method can be described as the “fast non touch” technique since the dissection is always kept 2 mm away from the arterial wall.
A standard median sternotomy is performed. Meticulous hemostasis of the sternal edges facilitates visibility during subsequent ITA harvesting. The prepericardial tissues are divided so that the pericardium, which is left intact during ITA harvesting, can be rapidly opened in case of hemodynamic instability. The left ITA is dissected first.
An internal thoracic artery sternal retractor is placed. The retrosternal areolar tissue and the pleural reflexion are opened with the Harmonic Synergy Curved Blade. Hemostasis and division of arterial branches or small vein tributaries can be achieved with it (or alternatively with electrocautery or the clip and cut method). The generator power is set at a minimum level of 3 and a maximum level of 5. The settings are tested for appropriate areolar tissue dissection and arterial coagulation and division.
When the pleura is opened, the ITA can usually be visualized, at least partly, and it can also be palpated. It lies at a variant distance laterally to the sternal edge, being almost parallel to it [17]. The satellite veins which are darker may help ITA location. The division of the endothoracic fascia starts at the upper third of the internal thoracic vessels, where there is usually more space between the internal thoracic artery and the medial internal thoracic vein. The endothoracic fascia along with the overlying parietal pleura are gently grasped with fine forceps just below the medial internal thoracic vein and are pulled down. With the lateral aspect of the ultrasonic blade tip the fascia is opened in-between the medial internal thoracic vein and the internal thoracic artery, being incised parallel to the vessels keeping the blade at least 2 mm away from the artery, not only to avoid arterial thermal trauma, but also to leave a thin layer or areolar tissue on the arterial wall.
After a longitudinal fascia opening is made and the posterior aspect of the artery is revealed, the dissection of the artery starts at a point where it appears best separated from the vein and the thoracic wall (usually the upper third). The artery is gently pulled away from the medial internal thoracic vein and the sternal wall, being picked up by small soft tissue remnants with fine forceps.
With minimal downward traction on the ITA, the belly of the scalpel is gently and quickly passed superiorly and parallel to it, dissecting the loose areolar tissue between the internal thoracic vein and the ITA. Care is taken to always keep the blade at a distance of at least 2 mm away from the artery and to avoid applying extensive tension on it. Loose adipose and connective tissue is cut and coagulated fast, disappearing with almost no resistance (cavitational fragmentation), revealing more dense structures such as the anteromedial aspect of ITA along with its branches, which are more resistant (the degree of resistance depending on their width.)
When an arterial branch becomes visualized it is further dissected proximally and distally to gain length for safe division at least 2 mm away from its origin at the arterial trunk. Occasionally, branch dissection can be performed using the curved blade tip, without using the ultrasonic function, with the scalpel curve properly oriented. While just passage is adequate for soft tissue dissection, branch division and simultaneous coagulation requires a few seconds. The lateral aspect of the blade is applied with slight tension perpendicularly to the branch, at the side of its wider angle with the arterial trunk, until spontaneous branch division.
At each arterial segment, the medial aspect is dissected first separating the artery from the medial vein, followed by anterior dissection separating the artery from the inner thoracic wall, and lateral dissection separating the artery from the lateral vein. The loose areolar tissue is dissected with the “fast non touch” method, while “close coagulation” and division of the branches is a slower procedure, requiring about 3 to 6 seconds, depending on the branch caliber. Most branches arise anteriorly or laterally but care should be taken for branches arising anteromedially. Arterial branches larger than 1.5 mm can be clipped and cut.
The dissection proceeds superiorly up to the subclavian vein and inferiorly up to the ITA bifurcation to the musculophrenic and the superior epigastric artery (or less commonly, the trifurcation to the previous branches and the diaphragmatic branch) [17].
To dissect the most proximal ITA segment and to divide the highest chest wall and pericardial branches, division of the medial internal thoracic vein is usually required. The vein (and perhaps its local tributaries) is dissected, clipped and divided by scissors.
During superior ITA dissection care should be taken to avoid injury to the phrenic nerve, which crosses the ITA most commonly anteriorly, but also posteriorly, at a varying distance from its origin from the subclavian artery (LITA mean 1.9 ± 0.7 cm (range 0.5 - 4.4 cm), RITA mean 1.5 ± 0 7 cm (range 0.3 to 4.5 cm)) [17]. It should be noted that in case of accidental touch of the operating blade to the phrenic nerve there is no diaphragmatic spasm, because there is no electricity conduction.
Care is taken to reveal and divide all superior internal thoracic artery branches, including the lateral costal branch. As an extra precaution to avoid coronary steal syndrome (and despite the argument that substantial diastolic flow reversal does not occur in the absence of significant proximal subclavian artery stenosis), the first anterior intercostal artery may be coagulated.
Opening of the endothoracic fascia and dissection of the arterial trunk and arterial branches are performed at power level 5 (maximum level providing faster cutting). Small and medium size arteries are also divided at high power level (5). The lateral side of the blade tip is applied to the branches for coagulation. Larger branches may require lower power level (2 or 3, providing greater coagulation). We rarely use the lower power level, preferring to clip large branches, particularly in hypertensive patients or patients with bleeding diathesis, such as patients receiving antiplatelet treatment preoperatively.
This method ensures an almost bloodless field, with minimal smoke production. There is no adherence of sticky burnt tissue material on the ultrasonic blade and there is no need for frequent blade cleaning or danger for unintentional artery pulling.
When the artery is fully dissected, it is rinsed with warm papaverine solution and wrapped in papaverine soaked gauze. If bilateral ITAs are used, the second ITA (usually the right) is dissected with the same method. Hemostasis of the chest wall is checked and ensured, usually with the Harmonic Curved Blade scalpel. In patients with bleeding diathesis meticulous hemostasis is required, and occasionally use of electrocautery or other hemostatic means.
Two clips are put to the peripheral end of the dissected arteries after systemic heparinization. The ITAs are divided distally before opening of the pericardium. The gross free ITA flow is estimated and the arteries are clamped with small atraumatic vascular clamps. The arteries are inspected for inadequate hemostasis, wall trauma, hematomas or discoloration, indicative of tear or dissection. Rarely a clip or a suture (8-0 polypropylene) may be needed. Suspicious or inadequate grafts are discarded.
Results
We have extensively harvested LITAs and BITAs with the Harmonic Synergy Curved Blade scalpel with an extremely low rate of harvesting damage, excellent graft quality as evaluated intraoperatively, sufficient RITA length for distal circumflex grafting, and extremely low rate of graft bleeding.
Cavitational fragmentation facilitates fast areolar tissue dissection protecting adjacent vascular structures, while branch coagulation and division is faster than clip and cut division, ensuring adequate hemostasis [21,22]. The first author has procured more than 2500 ITAs using this method in the last seven years. At transition from electrocautery skeletonization to ultrasonic scalpel skeletonization, the required time was substantially decreased. Having this experience, the harvesting time is currently about 15 minutes.
Discussion
The superiority of the ITA grafts over vein grafts for coronary revascularization, lying mainly in their superior long-term patency, led to a trend for extended arterial revascularization.
ITA patency by location of grafting
Large-scale retrospective studies showed superior ITA graft patency to that of vein grafts when an ITA was anastomosed to any left-sided coronary artery or to the posterior descending artery, at all times after CABG and with all degrees of proximal coronary stenosis. On the contrary, when bypassing the main right coronary artery (RCA) the early vein graft patency was equivalent or superior to that of ITA graft patency. However, at 10 years, the ITA graft patency was better when anastomosed to RCAs with a stenosis >/= 70% [13]. With careful patient selection and use of the second ITA to bypass an RCA with a 70% to 90% stenosis, free from distal stenoses and perfusing viable myocardium, similar survival was noted, independent of whether the second ITA was used to bypass the RCA or the circumflex system [14].
According to the current guidelines (ACCF/AHA 2011) arterial grafting of the RCA may be reasonable in the presence of critical stenosis (>/= 90%) (Class IIb, Level of Evidence: C). Arterial grafting of the RCA should not be performed in the absence of critical stenosis (< 90%) (Class C (harm), Level of evidence C). [2]
The most common approach in BITA revascularization is arterial grafting of the two most important left-sided arteries [14,15]. Thus, adequate ITA length that can be anastomosed without tension to diagonal and distant circumflex branches is an important graft quality parameter.
BITA versus SITA
Large-scale retrospective, nonrandomized comparative studies provided some indication that BITA grafting may be associated with higher survival, particularly during the second postoperative decade, and lower incidence of cardiac events, particularly repeat revascularization, in most patient subgroups. [6-12]
The large-scale multicenter randomized arterial revascularization trial (ART) showed low and similar 30-day (1%) and 1-year mortality (2.5%), and similar rates of stroke, myocardial infarction and repeat revascularization with BITA grafting to the two most important left-sided arteries versus SITA to LAD grafting (plus supplemental vein or radial artery grafting to the other diseased coronary arteries in both patient groups). BITA grafting resulted in increased length of operation (by 23 minutes) and increased duration of mechanical ventilation (by 103 minutes). Most importantly, BITA grafting was associated with increased incidence of sternal wound reconstruction (1.9% vs. 0.6%). Wound complication rate was higher among diabetic patients. Ten-year follow up data will provide more definitive evidence about the comparative clinical outcomes. It was suggested that patient selection and skeletonized rather than pedicled harvesting may be beneficial for reducing sternal wound complications. [15]
Operative techniques for ITA harvesting
The “pedicled” ITA harvesting is the conventional and most widespread technique. The ITA is taken down along with the surrounding tissue [15] including adipose tissue, the veins comitantes (the two accompanying, or satellite, internal thoracic veins), lymphatics, a stripe of endothoracic fascia, and muscle bands of the transversus thoracic muscle (transverse muscle of thorax) [16,17]). Harvesting is usually fast and is done with an electrocautery. This method preserves the structural integrity of IMA, but, particularly in BIMA grafting, decreases the sternal blood supply, and may result in wound complications especially in obese diabetic patients [1,15] and patients with respiratory impairment [15].
In semi-skeletonized harvesting the ITA is taken down along with the accompanying veins and the intercostal fat [16] as a thin pedicle without the endothoracic fascia and muscle bands, resulting in increased graft length [18]. This technique requires almost the same time and skill as the conventional wide pedicle technique [18]. Comparable rate of sternal wound complications was reported with the semi-skeletonized technique to those of other harvesting techniques [16].
Skeletonized IMA harvesting is a more recent and less extensively applied technique. The IMA is taken down isolated, free of all surrounding tissue. It is a more technically demanding and time-consuming method with a longer learning period. [19] The usual skeletonization method involves careful dissection with fine scissors and/or low voltage (or cold) electrocautery (diathermy) [19].
Skeletonized versus pedicled ITA harvesting
Potential advantages of the skeletonization technique in comparison to the pedicled technique include increased ITA graft length, minimization of inner thoracic wall trauma, reduction of intercostal nerve and vessel injury, preservation of collaterals and microcirculation, preservation of sternal perfusion and vein drainage, decreased risk of sternal wound infection, reduced need for transfusions, less pain and dysesthesia, preservation of pulmonary function, feasibility of visual inspection of the entire artery and timely identification of potential injury, increased ITA caliber, increased flow capacity and flow reserve. [19,26-33]. Due to minimal thoracic wall trauma and less sternal devascularization, skeletonized ITA and particularly BITA grafting may be advantageous in obese, diabetic patients and/or patients with respiratory impairment [1,15,28,29].
There are concerns, though, about the structural and functional integrity of the skeletonized grafts, regarding potential microscopic intimal dissection, endothelial lesion, microthrombi formation, external elastic lamina injury, deprivation of graft innervation, vasa vasorum perfusion, venous and lymphatic drainage, intimal hyperplasia, adventitia neorevascularization, altered vasoreactivity and endothelial function. [19,27]
Reviews and meta-analyses, including mainly case control studies and a few small scale randomized trials, provided some evidence supporting good quality of longer ITA grafts and decreased sternal wound complication rate with the skeletonization technique in comparison to the pedicled technique [19,26-29]. A review of small and medium scale retrospective or prospective non randomized studies showed at least comparable (and in 2 studies higher) short and mid term patency of skeletonized ITA grafts in comparison to that of pedicled ITA grafts, but long-term data is lacking [34]. A meta-analysis, including mainly case control studies and small scale randomized trials, showed at least similar early and mid term survival and incidence of cardiac events, and most importantly a potential benefit of skeletonization in mortality and cardiac morbidity in the high risk population [26], but the long-term results remain undetermined [19,26]. Thus, although there is weak evidence (level C) that there might be benefit with skeletonized grafting, there is no definitive data about the influence of the dissection method on patency, cardiac morbidity and mortality, particularly regarding the long-term outcomes. [19,26,27,34]
Ultrasonic ITA skeletonization
Higami et al [21] described in an experimental animal study that in ultrasonic ITA skeletonization (with the hook blade Harmonic scalpel, Ethicon, Endo-surgery) the low density adipose tissue planes adjacent to the artery are dissected with cavitational fragmentation without causing harm to artery (“by gently applying the blade tip to the fat tissue”, as if brushing it away). On the contrary the time required for ultrasonic coagulation and division of arterial branches is longer, being correlated with their outer diameter. When the blunt side of the hook blade was applied perpendicularly to the target branch (at level 2) the required time for safe coagulation and division was 2 – 3 seconds for branches 0.3 - 0.6 mm in diameter, and 3 - 4 seconds for branches 0.7 - 1.2 mm in diameter. They reported limited lateral spread of coagulation, with a length of about half of the depth of coagulation. For sufficient coagulation of branches 0.3 – 1.2 mm in diameter the mean length of intimal damage from the edge of the stump was only 0.60 mm (range 0.41 mm – 0.72 mm). They suggested that safe ultrasonic coagulation and division of human ITA branches 0.3 – 1.5 mm in diameter can be done at a distance of 1 mm from their origin from the main arterial trunk.
Higami et al [22,23] described a method for ultrasonic human ITA skeletonization with the hook type Harmonic scalpel (Ethicon Endo-Surgery). For fatty tissue dissection of the plane between the ITA and its satellite veins they used the “fast touch” method, sweeping the fatty tissue away (cavitational fragmentation), by lightly and quickly moving the ultrasonic scalpel along the ITA, to expose its adventitia, skeletonizing its main trunk safely and quickly.
In contrast, for treatment of the branches they used the “close coagulation” method which was a slower process. After exposing a branch, the blunt side of the hook blade was placed on it with averaged force compression, at least 1 mm away from the arterial trunk. The branch was sealed by protein coagulum at an output level of 2, within 3 to 4 seconds, and then it was spontaneously divided. They reported complete hemostasis, without injury to the main arterial trunk with this technique. [22,23]
Employing this technique clinically (n=200, mean age 64 years, 43% diabetes mellitus) Higami et al reported increased ITA graft length and increased free flow in comparison to those of pedicled grafts, and excellent 1-month and 1-year angiographic patency. They also reported no postoperative bleeding from the grafts and no mediastinitis with ultrasonic SITA or BITA (n=119, 60% of patients) harvesting. [23]
Ultrasonic skeletonization vs. pedicled harvesting
Matsumoto et al [35] reported that ultrasonic skeletonization preserved the smooth muscle function of ITA grafts, but compromised the endothelial function in comparison to pedicled harvesting.
Takami et al [25] in a small non randomized clinical study showed that ultrasonically skeletonized (Harmonic Scalpel, Ethicon Endo-Surgery, Cincinnati, OH) ITA grafts were associated to increased intraoperative graft diameter, and increased anastomotic graft flow (measured by the transit time flow meter) in comparison to pedicled ITA grafts. Early postoperative angiography showed excellent graft patency in both patient groups, with less degree of ITA narrowing to its peripheral third (close to the coronary anastomosis) with ultrasonic harvesting.
Pektok et al [36] in a small randomized trial showed no benefit of ultrasonic LITA skeletonization (Harmonic Scalpel; UltraCision, Inc; 5-mm twisted edge, HC 145, Level III) in comparison to 2 cm pedicled harvesting with a low setting electrocautery regarding the sternal perfusion on the seventh postoperative day. It should be noted though, that the study involved unilateral ITA harvesting, in relatively young patients, mainly men, with exclusion of diabetic and obese patients and patients with other risk factors for impaired wound healing, thus the results cannot be directly extrapolated to high risk patients, who may be more vulnerable to loss of collateral microcirculation.
Hirose et al [37] in a retrospective study of prospectively collected data suggested that ultrasonic BIMA skeletonized harvesting (with the hook type Harmonic scalpel, Ethicon Endo-Surgery) may be beneficial to pedicled harvesting in diabetic patients, decreasing wound complications and ensuring comparative clinical outcome and excellent postoperative angiographic patency.
Kinoshita at al [38] in a retrospective comparative study suggested that off-pump ultrasonically skeletonized (Harmonic Scalpel, Ethicon Endo-Surgery, OH) BIMA grafting of the left coronary system in elderly patients (>70 years) was associated with similar operative risk and decreased mid term overall mortality and cardiac event risk, in comparison to off-pump ultrasonically skeletonized single ITA grafting (and supplemental vein or gastroepiploic artery grafting in both groups). In contrast to existing evidence in pedicled BITA grafting, and despite they studied a patient population with fragile osteoporotic sternum, they found no significant difference in the incidence of sternal wound infection (1.4% BITA and 0.9% SITA, p=0.43). (Apart from the ultrasonic skeletonization, numerous other factors may have contributed to low sternal wound infection, including the off-pump method).
Ultrasonic vs. electrocautery or scissors skeletonization
Lamm et al [20] in a small scale clinical randomized trial showed superior histological endothelial integrity in ultrasonic ITA skeletonization (Ultrasonic (5 mm blade); Ethicon, Hamburg, Germany) in comparison to high frequency electrocautery close skeletonization (electrocautery Force 40; Valleylab, Boulder, CO, distance of the pedicle to the ITA wall < 0.5 mm). Disseminated loosening of subendothelial matrix cells was found if the ultrasonic scalpel was used immediately adjacent to the vessel with no instance of total loss of endothelial cells, while total loss of the vascular endothelium was found in all ITA samples with close electrocautery skeletonization. They reported carbonization damage at the outer ITA layer with close electrocautery skeletonization and absence of outer layer damage with the ultrasonic skeletonization. The authors commented that no irreversible endothelial damage was done at accidental contact of the ultrasonic blade to the artery.
Yoshikai et al [39] in a small non randomized clinical study showed histological endothelial integrity of ultrasonically skeletonized ITA grafts comparable to that of fine scissors skeletonized ITA grafts.
Canadas et al [40] confirmed these findings in a small clinical randomized trial comparing the graft quality of distally skeletonized ITAs either with the “fast touch” ultrasonic technique (harmonic scalpel, Ultracision Ethicon corp., Michigan) or with the scissors and clips technique. No endothelial disruption or basal membrane exposure was found in ultrasonic skeletonized harvesting. Similarly there was no damage in the case control group.
Urso et al [41] in a small randomized clinical trial showed significantly decreased flow of skeletonized ITA grafts after harvesting with both a low setting bipolar electrocautery and the ultrasonic scalpel (Harmonic Scalpel, Ethicon Endo-Surgery, Cincinnati, OH, branch coagulation and division at level 2). They reported no significant difference between groups (the flow was measured with the transit time flow meter at the beginning and at the end of harvesting). The flow reduction appeared reversible, being increased after graft anastomosis to the LAD. Decreased flow was attributed to manipulation related spasm, and was considered independent of the skeletonization technique.
Conclusions
The most widespread method for IMA harvesting is the pedicled technique and most studies showing excellent long-term patency of ITA grafts concern mainly pedicled grafts.
The skeletonization technique may be beneficial, causing less thoracic wall surgical trauma and providing increased graft length to allow revascularization of distant circumflex branches. The skeletonization technique is more technically demanding and time-consuming having a longer learning period. Furthermore, there are concerns about the quality of the arterial grafts after skeletonized harvesting, while evidence is lacking regarding their long-term patency. The quality of ITA grafts is of outmost importance, for their short and long-term patency, which is related to survival.
Skeletonized IMA harvesting can be beneficial in reducing sternal wound complications, particularly in bilateral harvesting, but also in unilateral harvesting especially in high risk patients such as diabetic patients or patients with other microvasculopathy, and/or obese patients, and/or older patients with thin osteoporotic sternum, and/or patients with compromised respiratory function [15,28,29]. Ultrasonic skeletonized harvesting may further increase the benefit by reducing the inner thoracic wall thermal injury [23,37,38].
The ultrasonic scalpel may be beneficial in optimizing the quality of skeletonized ITA grafts, particularly if it is difficult to keep a wide distance (>0.5 mm) during harvesting [20,25]. Ultrasonic skeletonization with the “fast touch” technique may be beneficial in preserving the functional and structural ITA integrity [20,22,23,25,39,40], thus ensuring good short and long-term patency. Ultrasonic skeletonization with the described and video illustrated “fast non touch” technique may be beneficial by further reducing arterial thermal trauma. Further evidence is required to positively confirm the superiority of ultrasonic skeletonized harvesting over other harvesting methods.
VIDEO
References
- Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2004;44:1146-310.
- Hillis LD, Smith PK, Anderson JL, et al, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2012;143(1):4-34.
- Antman, EM, Anbe, DT, Armstrong, PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Circulation. 2004;110;588-636.
- Loop FD,Lytle BW,Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1-6.
- Cameron A, Davis KB, Green G, Schaff HV. Coronary bypass surgery with internal thoracic-artery grafts: effects on survival over a 15-year period. N Engl J Med. 1996;334:216-9.
- Taggart DP, D'Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358(9285):870-5.
- Lytle BW, Blackstone EH, Loop FD, et al. Two internal thoracic artery grafts are better than one. J Thorac Cardiovasc Surg. 1999;117(5):855-72.
- Lytle BW, Blackstone EH, Sabik JF, et al. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg. 2004;78:2005-12
- Stevens LM, Carrier M, Perrault LP, et al. Single versus bilateral internal thoracic artery grafts with concomitant saphenous vein grafts for multivessel coronary artery bypass grafting: effects on mortality and event-free survival. J Thorac Cardiovasc Surg. 2004;127:1408-15.
- Burfeind WR Jr, Glower DD, Wechsler AS, et al. Single versus multiple internal mammary artery grafting for coronary artery bypass: 15-year follow-up of a clinical practice trial. Circulation. 2004;110(11 Suppl 1):II27-35.
- Kurlansky PA, Traad EA, Dorman MJ, et al. Thirty-year follow-up defines survival benefit for second internal mammary artery in propensity-matched groups. Ann Thorac Surg. 2010;90(1):101-8.
- Galbut DL, Kurlansky PA, Traad EA, et al. Bilateral internal thoracic artery grafting improves long-term survival in patients with reduced ejection fraction: A propensity-matched study with 30-year follow-up. J Thorac Cardiovasc Surg. 2012;143(4):844-853.e4.
- Sabik JFI, Lytle BW, Blackstone EH, et al. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann Thorac Surg. 2005;79:544-51.
- Sabik JF 3rd, Stockins A, Nowicki ER, et al. Does location of the second internal thoracic artery graft influence outcome of coronary artery bypass grafting? Circulation. 2008 30;118(14 Suppl):S210-5.
- Taggart DP, Altman DG, Gray AM, et al.; ART Investigators. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART). Eur Heart J. 2010;31(20):2470-81.
- Deng Y, Byth K, Paterson HS. Semi-skeletonized internal mammary artery grafts and sternal wound complications. Asian Cardiovasc Thorac Ann. 2004;12(3):227-32.
- Henriquez-Pino JA, Gomes WJ, Prates JC, Buffolo E. Surgical Anatomy of the Internal Thoracic Artery. Ann. Thorac Surg. 1997;64(4):1041-5.
- Horii T, Suma H. Semiskeletonization of internal thoracic artery: alternative harvest technique. Ann Thorac Surg. 1997;63(3):867-8.
- Athanasiou T, Crossman MC, Asimakopoulos G, et al. Should the internal thoracic artery be skeletonized? Ann Thorac Surg. 2004;77(6):2238-46.
- Lamm P, Juchem G, Weyrich P, et al.The harmonic scalpel: optimizing the quality of mammary artery bypass grafts Ann Thorac Surg. 2000;69:1833-1835
- Higami T, Maruo A, Yamashita T, et al. Histologic and physiologic evaluation of skeletonized internal thoracic artery harvesting with an ultrasonic scalpel. J Thorac Cardiovasc Surg. 2000;120(6):1142-7.
- Higami T, Kozawa S, Asada T, et al. Skeletonization and harvest of the internal thoracic artery with an ultrasonic scalpel. Ann Thorac Surg. 2000;70(1):307-8.
- Higami T, Yamashita T, Nohara H, et al. Early results of coronary grafting using ultrasonically skeletonized internal thoracic arteries. Ann Thorac Surg 2001;71:1224–8.
- Kullar PJ, Sorenson K, Weerakkody R, Adams J. The management of advanced oral cancer in a Jehovah’s Witness using the Ultracision Harmonic Scalpel. World J Surg Oncol. 2011; 9: 115
- Takami Y, Ina H. Effects of skeletonization on intraoperative flow and anastomosis diameter of internal thoracic arteries in coronary artery bypass grafting. Ann Thorac Surg. 2002;73:1441-1445.
- Hu X, Zhao Q. Skeletonized internal thoracic artery harvest improves prognosis in high-risk population after coronary artery bypass surgery for good quality grafts. Ann Thorac Surg. 2011;92(1):48-58.
- Raja SG, Dreyfus GD. Internal thoracic artery: to skeletonize or not to skeletonize? Ann Thorac Surg. 2005;79(5):1805-11.
- Behranwala AA, Raja SG, Dunning J. Is skeletonised internal mammary harvest better than pedicled internal mammary harvest for patients undergoing coronary artery bypass grafting? Interact Cardiovasc Thorac Surg. 2005;4(6):577-82.
- Saso S, James D, Vecht JA, et al. Effect of skeletonization of the internal thoracic artery for coronary revascularization on the incidence of sternal wound infection. Ann Thorac Surg. 2010;89(2):661-70.
- Bonacchi M, Prifti E, Giunti G, Salica A, Frati G, Sani G. Respiratory dysfunction after coronary artery bypass grafting employing bilateral internal mammary arteries: the influence of intact pleura. Eur J Cardiothorac Surg 2001;19: 827–33.
- Cohen AJ, Lockman J, Lorberboym M, et al. Assessment of sternal vascularity with single photon emission tomography after harvesting of the internal thoracic artery. J Thorac Cardiovasc Surg 1999;118:496–502.
- Mannacio V, Di Tommaso L, De Amicis V, et al. Randomized flow capacity comparison of skeletonized and pedicled left internal mammary artery. Ann Thorac Surg. 2011;91(1):24-30.
- Kamiya H, Akhyari P, Martens A, et al. Sternal microcirculation after skeletonized versus pedicled harvesting of the internal thoracic artery: A randomized study. J. Thorac. Cardiovasc. Surg., 2008; 135: 32 - 37.
- Ali E, Saso S, Ashrafian H, Athanasiou T. Does a skeletonized or pedicled left internal thoracic artery give the best graft patency? Interact Cardiovasc Thorac Surg. 2010;10(1):97-104.
- Matsumoto K, Tsuneyoshi I, Iguro Y, et al. Effects of ultrasonic skeletonization on internal thoracic and gastroepiploic arteries for coronary artery bypass grafting. Eur J Cardiothorac Surg. 2006;30(4):592-6.
- Pektok E, Cikirikcioglu M, Engin C, et al. Does harvesting of an internal thoracic artery with an ultrasonic scalpel have an effect on sternal perfusion? J Thorac Cardiovasc Surg. 2007;134(2):442-7.
- Hirose H, Amano A, Takanashi S, Takahashi A. Skeletonized bilateral internal mammary artery grafting for patients with diabetes. Interact Cardiovasc Thorac Surg. 2003;2(3):287-92.
- Kinoshita T, Asai T, SuzukiT, Kuroyanagi S, et al. Off-pump Bilateral Skeletonized Internal Thoracic Artery Grafting in Elderly Patients. Ann. Thorac. Surg., 2012; 93: 531 - 536.
- Yoshikai M, Ito T, Kamohara K, Yunoki J. Endothelial integrity of ultrasonically skeletonized internal thoracic artery: morphological analysis with scanning electron microscopy. Eur J Cardiothorac Surg. 2004;25(2):208-11.
- Lima Cañadas PP, Cañas AC, Orradre Romeo JL, et al. Endothelium histological integrity after skeletonized dissection of the left internal mammary artery with ultrasonic scalpel. Interact Cardiovasc Thorac Surg. 2005;4(3):160-2.
- Urso S, Alvarez L, Sádaba R, Greco E. Skeletonization of the internal thoracic artery: a randomized comparison of harvesting methods. Interact CardioVasc Thorac Surg, 2008 7(1): 23-26



