ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Massive Left Ventricular Aneurysm
Introduction
Left ventricular aneurysm (LVA) is defined as circumscribed, thin-walled, non-contractile out-pouching of the ventricle (1). True aneurysm of left ventricle (LV) develops after completed myocardial infarction resulting in the out-pouching of thinned and scarred myocardium which becomes dyskinetic in systole. LV aneurysms predispose to thrombo-embolism, congestive cardiac failure, and ventricular arrhythmias (2).
Anterior wall aneurysms are by far more common, while only 3% of LV aneurysms are inferior. LV aneurysms are often clinically silent and diagnosed on routine imaging. Otherwise patients can present with symptoms of heart failure, embolic event, or ventricular arrhythmia. Persistent ST-elevation on 12 lead-ECG may be seen, but has a low sensitivity and specificity for the presence of aneurysm. Further imaging is mandatory in aneurysm management (2).
True LV aneurysm can be managed medically, in contrast LV pseudoaneurysm requires urgent surgical resection as the risk of rupture is high. Therefore, differentiating between true LV aneurysm and LV pseudoaneurysm is essential and modern imaging modalities like cardiac CT or MRI are indispensable.
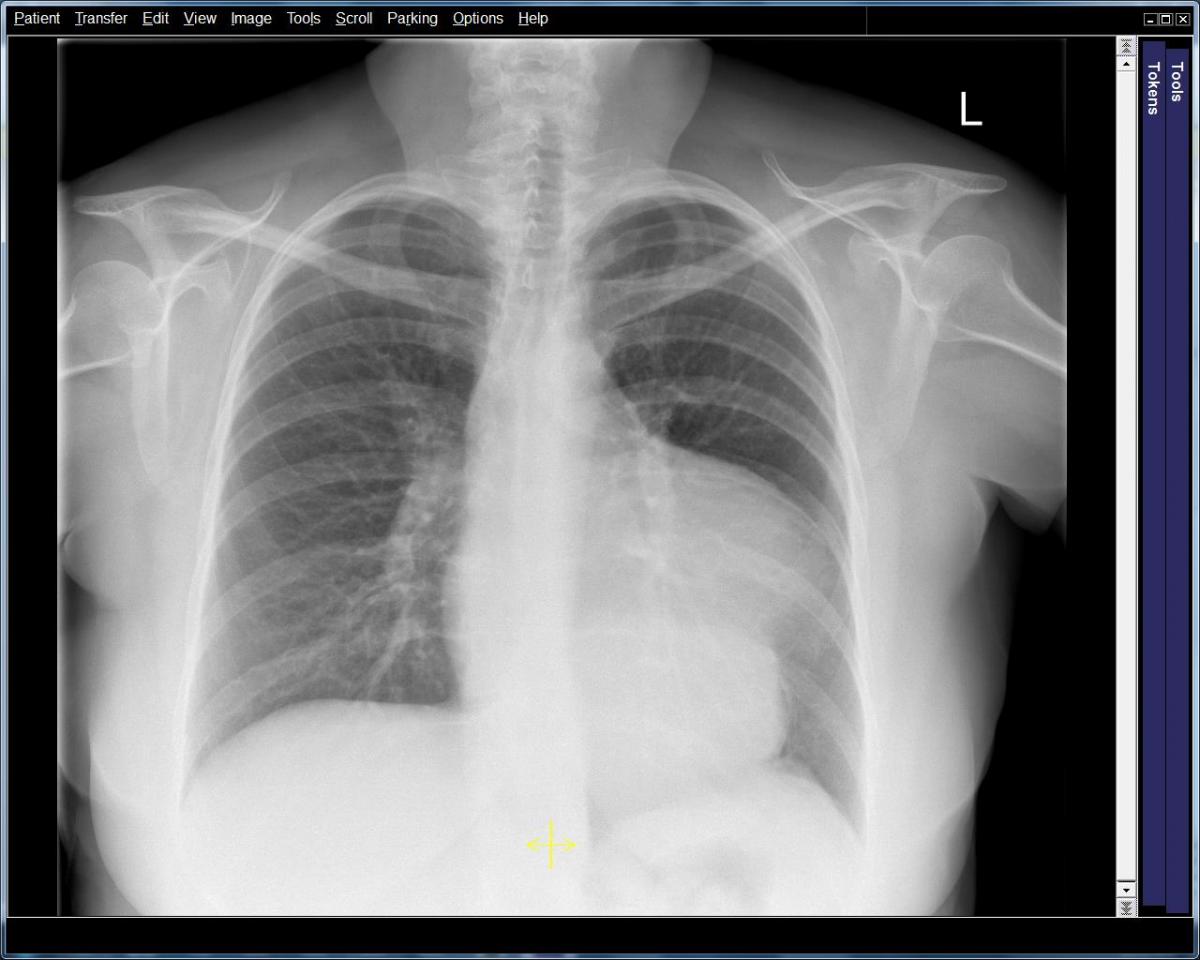
Figure 1: PA Chest radiograph revealing a soft tissue density measuring 6.7 x 9 cm, merging with the left heart border. Most likely vascular in origin, however mediastinal masses could not be ruled out.
Case Report

Figure 2: Organised thrombus removed from aneurysmal sac.
A 63-year-old female presented with a large inferior myocardial infarction. She had been found at home, collapsed, with signs and symptoms of a cerebrovascular event and a recent myocardial infarction on 12 lead ECG.
A transthoracic echo (TTE) performed on the same day showed impaired Left Ventricle (LV) systolic function with severe hypokinesia of the inferior wall. She was also noted to have a mass present attached to the mid inferior wall with echogenicity similar to the myocardium, approximately 1cm in width. This was diagnosed as an LV thrombus and she was subsequently anti-coagulated.
The patient’s neurological symptoms continued to improve with only minor residual weakness in her left arm. She began to demonstrate signs of progressive cardiac failure. Subsequent serial TTE demonstrated a progressively enlarging inferior aneurysm. Coronary angiography showed her to have an occluded right coronary artery. Chest radiography and CT scan of the thorax showed a very large aneurysmal dilatation of the left ventricle containing a moderate amount of thrombus (Figure 1). The aneurysm arose from the inferior wall of the mid cavity of the ventricle. Its neck measured approximately 40 mm. The aneurysm itself measured 110 x 90 x 120 mm. The left ventricle was dilated with thinning of the myocardium globally. In contrast, the cardiac MRI (Video 1,2) showed that the left ventricle had a ruptured inferior free wall measuring 30 x 32 mm, which opened into an inferior pseudoaneurysm measuring 60 mm deep and 99 mm across, with a significant layer of thrombus.
The patient underwent left ventricular aneurysmectomy. Standard median sternotomy was used and the patient was placed onto cardiopulmonary bypass via a two stage venous cannula in the right atrium. An aortic clamp was applied and cold blood cardioplegia was infused into the aortic root. With the heart arrested, the inferior aneurysm was dissected and opened, and approximately 250 grams of thrombus was removed. The neck of the aneurysm was demarcated (30 mm wide by 50 mm long), and was oversewn with a sauvage Dacron patch using 2.0 prolene (Figure 3). This was reinforced with Teflon pledgeted 3.0 prolene interrupted sutures. The remaining aneurysm sac was removed and closed with Teflon to buttress over the patch with a 1.0 Prolene Carrel stitch.
The patient was weaned off cardiopulmonary bypass (CPB) with low dose inotropic support. Transoesophageal echocardiography after weaning off CPB demonstrated reasonable LV function with slight dyskinesia of the septum (secondary to DDD pacing) but well-functioning anterior and lateral walls. The inferior wall remnant remained akinetic.
The patient made an uneventful post-operative recovery and was discharged home day 7 with no further neurological or cardiovascular sequelae.
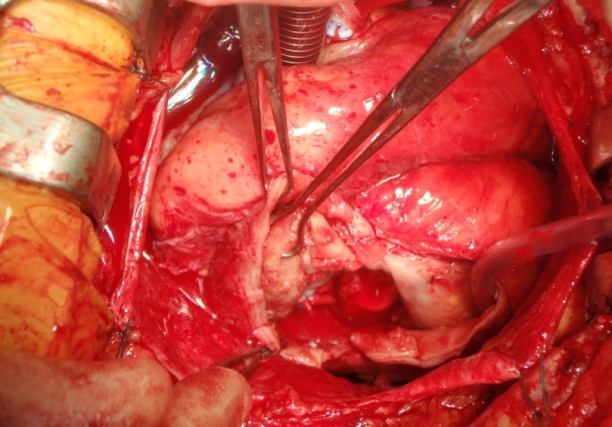
Figure 3: View through incised sac. Sauvage dacron patch in place over aneurysmal neck.
Discussion
Transthoracic echocardiography plays an important role and provides assessment of LV structure and function, however, inferior wall aneurysm can be difficult to visualize particularly in patients with sub-optimal image quality. In patients with poor acoustic windows, contrast echocardiography is useful in identifying presence of aneurysm. Transoesophageal echocardiography has a limited role due to its semi-invasive nature and the availability of superior non-invasive imaging modalities like cardiac MRI and CT (3).
Cardiac CT offers complementing information regarding the anatomy of aneurysm as well as that of coronary arteries, though at an expense of ionizing radiation. Cardiac MRI provides a comprehensive assessment of the morphology of the aneurysm and adjacent myocardium. Furthermore, it demonstrates the continuity of the myocardial wall, thus differentiating between a real and a pseudo- aneurysm. If surgical repair is being considered, the identification of viable myocardium is important and tissue characterization by MRI delivers additional key information not available with other techniques. Furthermore, the dynamic nature of MRI scanning and its ability to quantify LV ejection fraction, LV volumes, and mitral regurgitation allow operative planning and appropriate risk stratification (2).
Management of aneurysm largely depends on presenting symptoms and nature of aneurysm i.e. true aneurysm or pseudoaneurysm. Perceived high risk of spontaneous rupture associated with pseudoaneurysm and its catastrophic consequences dictate urgent surgical repair whereas true LV aneurysm can be managed both surgically and medically. Medical management focuses on reducing the risk of embolism and treating underlying congestive cardiac failure. The aim of surgical therapy is restoration of LV geometry, LV volume reduction, and the relief of ischaemia by CABG in the presence of concomitant coronary artery disease in viable myocardial territory (4).
Conclusion
This patient demonstrates that not all pseudo-aneurysms rupture rapidly. Despite this, we would still agree that early diagnosis of pseudo-aneurysm mandates urgent surgery. Furthermore, she shows that a number of different imaging modalities are often needed in the subsequent investigation and management of patients who develop cardiac failure after myocardial infarction.
Video 1: Cardiac MRI demonstrating an inferior left ventricular pseudo aneurysm 60 mm deep and 99 mm across with significant layer of thrombus, neck measures approximately 42 mm.
Video 2: Cardiothoracic MRI demonstrating extent of thrombus.
References
- Hamer DH, Lindsay J, Jr. Redefining true ventricular aneurysm. Am J Cardiol 1989;64:1192-4.
- Heatlie GJ, Mohiaddin R. Left ventricular aneurysm: comprehensive assessment of morphology, structure and thrombus using cardiovascular magnetic resonance. Clin Radiol 2005;60:687-92.
- Haddadian B, Jan MF, Paterick TE, Khandheria BK, Tajik AJ. Multimodality imaging of left ventricular aneurysm: tools of the trade. Eur Heart J Cardiovasc Imaging;13:629.
- Toker ME, Onk OA, Alsalehi S, et al. Posterobasal left ventricular aneurysms: surgical treatment and long-term outcomes. Tex Heart Inst J;40:424-7.




Comments