ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Resection of Symptomatic, Complex Aspergilloma
Patient Selection
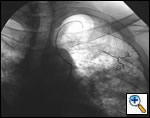
The definitive treatment of symptomatic aspergilloma is surgical resection. Patients with aspergilloma who present with hemoptysis are at risk of death due to massive hemoptysis and/or respiratory insufficiency caused by aspirated blood. Catheter embolization of bronchial arteries can be used to control acute bleeding (Figure 1), but is not durable. Likewise, intracavitary instillation of antifungal agents has mixed outcomes and seldom results in complete resolution of the cavity (Figure 2 ) 1. Systemic antifungal agents play little role as primary treatment because they do not penetrate the aspergilloma cavity 2. Resection in appropriate patients provides a definitive diagnosis and a durable treatment response.
 |
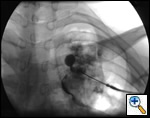 |
| Figure 1: Catheter embolization of bronchial arteries | Figure 2: Intracavity instillation of antifungal agents |
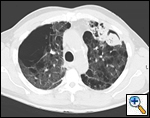 |
| Figure 3: A complex aspergilloma |
Unfortunately, most patients with aspergilloma have significant underlying pulmonary disease and are at increased risk for resection. An aspergilloma in the setting of underlying pulmonary disease is referred to as a complex aspergilloma (Figure 3). Associated pulmonary diseases in our practice have included bronchiectasis, sarcoidosis, COPD, lung abscess, rheumatoid lung disease, tuberculosis, and complications of systemic immunosuppression.
In general, we do not operate on patients who have active and massive hemoptysis. Instead, the airway is secured and conservative attempts (selective intubation, balloon tamponade, embolization) are use to control bleeding. Once the patient is stabilized and the bleeding has stopped, rigid bronchscopy is performed to clear the airway of all clots. The patient is then evaluated for resection in a more elective manner during the same hospitalization.
As with any candidate for major pulmonary resection, evaluation begins with spirometry. Those patients with severe underlying lung disease also require perfusion scan. This study often reveals reduced perfusion in the diseased portions of lung, suggesting that even patients with poor overall lung function can tolerate resection when the areas of poor perfusion match the areas to be removed. Nutritional status must also be evaluated and supplemented as these patients are often malnourished. There is no well-established cutoff for FEV1 or calculated postoperative FEV1 to determine a patient’s candidacy for resection. The FEV1 and perfusion scan are considered along with the patient’s medical condition and the surgeon’s experience to determine operability. Patients felt to be at prohibitive risk for resection can undergo repeated percutaneous injection of amphotericin gel as a temporizing measure. Although cavernostomy is widely discussed in the literature, it is associated with mortality rates of 30% and has not been performed in our institution during the last decade.
Operative Steps
 |
| Figure 4: Serratus and lattissimus muscles on pedicle flaps |
The operative plan is to remove the aspergilloma by performing an extrapleural lobectomy with avoidance of pleural contamination, obliterate the residual space, and prevent bronchopleural fistulae. A thoracic epidural catheter is placed by the anesthesia team for intra- and postoperative pain management. A posterolateral incision is made and subcutaneous flaps are raised over the lattissimus dorsi and serratus muscles. The serratus and lattissimus muscles are raised as pedicle flaps based on the thoracodorsal artery (Figure 4). Currently, we use muscle transposition in all patients undergoing resection of complex aspergilloma. The transposed muscles are used to buttress the bronchial stump and to obliterate residual pleural space if present.
The chest is entered through an extrapleural plane in the 5th intercostal space. If required, the fifth rib is removed. Chest wall bleeding can be significant and is controlled with either the TissueLink bipolar radiofrequency device or an argon beam coagulator. Once chest wall hemostasis is confirmed, attention is turned toward the hilum.
Proximal pulmonary arterial control is obtained in all patients undergoing major lung resection with inflammatory diseases. This provides a significant level of safety during resection of inflamed, friable tissue where major segmental arterial branches can easily be injured. The mediastinal pleura is incised and the ipsilateral pulmonary artery is dissected circumferentially. A Semb clamp is used to pass an umbilical tape around the artery. This is secured with a loose Rommell type tourniquet and is available should emergency vascular control be needed during the procedure.
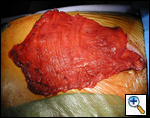
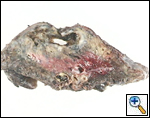
A standard lobectomy is performed. Dissection in the fissure is avoided as this will only lead to prolonged postoperative air leak. Instead, once the arterial anatomy has been defined, the fissures are completed with a stapling device. In rare instances the pulmonary parenchymal tissue is too thick to staple; in those cases, it must be taken with a clamp, cut, and then over sewn with chromic suture. A resected specimen is shown in Figure 5 along with the micrograph showing the typical appearance of recovered fungal elements (Figure 6).
 |
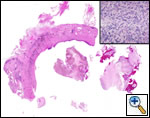 |
| Figure 5: A resected specimen | Figure 6: Appearance of recovered fungal elements |
To prepare for closure, an additional incision is made through the intercostal muscle of the second or third interspace. The muscle pedicles are brought into the chest through these openings. The intercostal space should be opened widely to avoid any compression of the vascular bundle supplying the transposed muscle. On occasion, a partial rib resection is performed to allow for additional space, but we have not found it necessary to do this on a routine basis. The bronchial closure is buttressed with muscle secured to the stump with 4-0 Maxon suture. The remaining muscle is used to obliterate all residual space. Two 32 Fr pleural chest tubes are placed. The ribs are reapproximated with #1 Vicryl sutures. Multiple 10 mm Jackson Pratt drains are placed in the subcutaneous muscle harvest sites and brought out through separate stab incisions. The subcutaneous tissues and skin are closed in layers. The Jackson Pratt drains are placed to wall suction for the first 48 hours and then converted to bulb suction.
Every attempt is made to extubate the patient in the operating room. Following extubation, the patients are transferred to a thoracic surgical floor with specialized nursing and respiratory therapy care. Early mobilization and aggressive pulmonary toilet are of paramount importance. Most patients are discharged with the Jackson Pratt drains in place. The drains are removed in the outpatient clinic once the drainage is less than 30 cc per 24 hours. Physical therapy is usually begun 4 to 6 weeks after the operation to restore shoulder strength and flexibility lost by muscle harvest.
Preference Card
- Standard thoracotomy tray with rib instruments.
- Deep narrow Deaver retractors to aid in muscle harvest.
- Semb clamp to pass umbilical tape around proximal PA.
- Bipolar TissueLink RF device (TissueLink Medical, Inc. Dover NH) or Argon beam coagulator for chest wall bleeding.
Tips & Pitfalls
- Avoid operating on these difficult patients as an emergency.
- Careful patient selection is important; not every patient qualifies for surgery.
- Prepare muscle pedicles prior to opening the chest.
- Obtain proximal control of the pulmonary artery before beginning hilar dissection.
- Control chest wall bleeding early to avoid creating an unstable situation.
- Use pedicled muscle flaps routinely to reinforce bronchial closure, seal parenchymal air leaks, and eliminate pleural space problems.
Results
Early large surgical reviews reported overall operative mortality rates as high as 25% and morbidity rates of 60% 3. Reviews published more recently report 0 to 6% operative mortality rates 4, 5, and morbidity rates of 18% 6. These results are probably due to a combination of better patient selection, modified operative techniques, an improved postoperative management.
It appears that the morbidity and mortality associated with resection of complex aspergilloma results from four major factors: (1) the underlying lung disease, (2) intraoperative hemorrhage, (3) prolonged air leaks, and (4) incomplete reexpansion of the remaining lung with postoperative space complications. Unfortunately, little can be done to improve the patient’s underlying lung function. Instead, the surgeon must scrutinize the preoperative testing and exercise careful judgment in determining which patients will tolerate resection. Intraoperative hemorrhage can be controlled with meticulous attention to chest wall hemostasis as well as the routine use of proximal pulmonary artery control. The routine transposition of pedicled muscle flaps addresses both the problem of prolonged air leaks and residual pleural space.
Our recent institutional experience with surgical resection for complex pulmonary aspergilloma includes 22 consecutive patients over a thirteen year period. Preoperative FEV1 ranged from 20% to 80% of predicted. We performed 5 pneumonectomies, 11 lobectomies, 2 segmentectomies, and 2 wedge resections. There were two in-hospital deaths. One patient had minimal postoperative hemoptysis, likely due to underlying lung disease. A total of twelve patients had transposition of pedicled muscle flaps. This technique was used in the more recent patients in our series and was associated with lower overall morbidity.
Review of the literature confirms overall improved outcomes in patients with complex aspergilloma undergoing resection. In 2000, Regnard, et al., performed a literature analysis specifically evaluating the operative mortality in complex forms of aspergilloma to be used for comparison against their own series 5. They found earlier reviews by Daly 3 and Stamatis 7 had operative mortality rates of 18% and 34% respectively. Their operative mortality rate in this report was 6%.
Surgical resection remains the definitive treatment of symptomatic complex aspergilloma. Improvement in patient selection, operative techniques, and postoperative care has markedly improved the outcome in these very difficult patients.
References
- Giron J, Poey C, Fajadet P, et al. CT guided percutaneous treatment of inoperable pulmonary aspergillomas: a study of 40 cases. Eur J Radiol 1998;28:235-242. Cited in PubMed; PMID: 9881259 [Citation]
- Stevens DA, Kan VL, Judson MA, et al. Practice guidelines for diseases caused by Aspergillus. Clin Infect Dis 2000;30:696-709. doi: 10.1086/313756 [Full Text]
- Daly RC, Pairolero PC, Piehler JM, Trastek VF, Payne WS, Bernatz PE. Pulmonary Aspergilloma: Results of surgical treatment. J Thorac Cardiovasc Surg 1986;92:981-988. [Abstract]
- Babatasi G, Massetti M, Chapelier A, et al. Surgical treatment of pulmonary Aspergilloma: Current outcome. J Thorac Cardiovasc Surg 2000;119:906-912. [Abstract] [Full Text]
- Regnard JF, Icard P, Nicolosi M, et al. Aspergilloma: A series of 89 surgical cases. Ann Thorac Surg 2000;69:898-903. [Abstract]
- Chen JG, Chang YL, Luh SP, Lee JM, Lee YC. Surgical treatment for pulmonary Aspergilloma: a 28 year experience. Thorax 1997;52:810-813. doi 10.1136/thx.52.9.810 [Abstract]
- Stamatis G, Greschuchna, D. Surgery for pulmonary Aspergilloma and pleural Aspergillosis. Thorac Cardiovasc Surg. 1988 Dec;36(6):356-60. Cited in PubMed; PMID: 3068829 [Citation]



