A video is available below that demonstrates Phases II - IV of robotic thoracoscopic first rib resection.
Patient Selection
Robotic thoracoscopic first rib resection is indicated in patients diagnosed with Paget-Schroetter disease. In our patients, we have confirmed the diagnosis by clinical history, physical exam with dynamic upper extremity maneuvers (Adson and Wright tests), and dynamic venography. This technique is also feasible in patients who have undergone incomplete rib resection by conventional techniques.
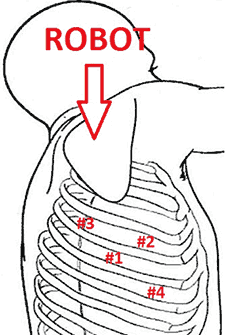
Figure 1. Port placement and robot positioning for robotic first rib resection. Numbers 1,2,3, and 4 indicate incisions.
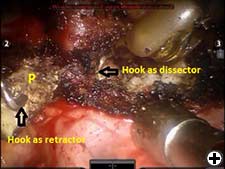
The operation is performed on a video-assisted thoracoscopic surgery (VATS) platform. The robot is used to dissect the first rib and divide the scalene muscles. The procedure is performed in four phases. The patient is placed in the lateral decubitus position with the affected side up and hips slightly flexed with minimal reverse Trendelenberg. An anesthesiologist with experience should be managing the airway because of limited control during the robotic part of the operation. Single-lung ventilation is used to facilitate exposure and dissection. Three 2cm incisions are made for camera and working ports. Incision #1 is made in 5th intercostal (IC) space at midaxillary line; this incision will be used for camera placement. Incision #2 is made in 4th IC space at the anterior axillary line. Incision #3 is made in the 4th IC space at the posterior axillary line. One 1cm incision is made both for retractor (Endopaddle Retract; Auto Suture, Covidien, Mansfield, Ma) placement and postoperative chest tube placement. This incision is made in the 6th IC space at the anterior axillary line (Figure 1). The robot (da Vinci, Intuitive Surgical, Sunnyvale, CA) is brought in from the head of the bed. The camera robotic arm is placed in incision #1. The right robotic arm with hook cautery is placed in incision #2. The left robotic arm with an endograsper is placed in incision #3. It is crucial to identify the following structures: subclavian vein, subclavian artery, first rib, second rib, sympathetic chain, phrenic nerve, and the superior vena cava (Figure 2). Hook cautery is used to dissect the parietal pleura and periostium off the rib, outlining the first rib. The dissection is carried medially to the costosternal junction, laterally to the vertebral body, anteriorly to the border of the rib, posteriorly to the border of the rib, and superiorly 3mm onto the upper surface of the rib in a cephalad extrathoracic direction (Figure 3). Dissect the borders of rib enough so that the borders of the subclavian artery, vein, and rib can clearly be distinguished. The thickness of the rib is assessed and the area between the subclavian vein and artery is isolated. This area represents the thinnest portion of the first rib and is most suitable for an osteotomy. In this phase, the robotic arms are withdrawn and the rib is cut between the subclavian vein and artery using a 6mm thoracoscopic Kerrison bone cutter (Depuy, Raynham, Ma). This area represents the thinnest portion of the first rib. Division of the rib at its midpoint allows for the rib to be pivoted on the sternocostal joint in a trap door configuration (Figure 4). Figure 2. Intraoperative photograph showing the location of the important anatomical structures. 1- first rib, 2- second rib, SA- subclavian artery, SV- subclavian vein, SVC- superior vena cava. Figure 3. Intraoperative photograph demonstrating the extent of dissection outlining the first rib. L-lateral, M-medial, A-anterior, P-posterior. Figure 4. The divided first rib. The rib is made into a trap door configuration pivoting at the sternocostal joint. L-lateral, M-medial, A-anterior, P-posterior. Figure 5. Posterior dissection of the first rib. One hook cautery is used for dissection while the other hook cautery is used for retraction. P-posterior. A hook cautery is placed in both robotic arms. The arms are replaced in their original positions. In an alternating fashion, depending on the anatomy, the hook is used for retraction as well as cautery dissection of the first rib (Figure 5). Dissection is continued with the hook cautery, staying intimate with the first rib to avoid injury of nearby neurovascular structures. Scalenus anticus and medius are subsequently divided from the first rib. As the first rib is freed from its surrounding tissues, the first rib is retracted in a trap door configuration to aid in dissection at the costosternal joint. Once adequate dissection has occurred, the rib is disarticulated from its respective joint. The robot is removed and the rib is placed in a large endobag (Endopouch specimen retrieval bag (10mm diameter), Ethicon) and extracted from the thoracic cavity through a 2cm port. The retractor is removed from incision #4 and a 24Fr chest tube is placed through this incision. The lung is reinflated and the incisions are closed. There were 13 first rib resections in 9 patients. 4 patients underwent staged bilateral first rib resection. There were 6 men and 3 women. Mean age was 34 +/- 8.5 years. At the time of initial presentation, on ultrasonography the subclavian vein was thrombosed on the right side in 7 patients and left side in 2 patients. In 4 patients who underwent staged bilateral first rib resection, surgery was performed prior to subclavian vein thrombosis. In 8/9 (89%) patients, subclavian vein thrombosis occurred with activity involving the upper extremity. In 1/9 (11%) patients, subclavian vein thrombosis was associated with an indwelling catheter. Prior to referral for surgery, all patients were anticoagulated. On physical exam, all patients had swelling of the affected arm. All operated extremities demonstrated positive Adson and Wright signs. All patients were evaluated for underlying coagulation disorders. Coagulation studies were negative in 5/9 patients (56%). Factor V Leiden deficiency was seen in 1/9 patients (11%). Mutation in the MTHFR gene was diagnosed in 3/9 patients (33%). Prior to surgical intervention, static upper extremity venography showed an open subclavian vein in 7/9 (78%) patients and an occluded subclavian vein in 2/9 (22%) patients. On hyperabduction of the affected extremity, there was occlusion of the vein at the sternocostal junction in 7/9 (78%) patients. In the 2 patients with an occluded subclavian vein, there was engorgement of the collateral vessels with hyperabduction of the affected extremity. Upper extremity venography of the unaffected side showed a patent subclavian vein in 9/9 (100%) patients. However, hyperabduction of the unaffected extremity showed partial occlusion of the vein in all patients. The first rib was removed en bloc. In all patients, a bony protuberance was noted immediately lateral to the sternocostal joint. In 2/13 (15%) specimens the bony protuberance articulated with the underside of the clavicle just lateral to the sternocostal joint. In 1/13 (8%) specimens the junction of the bony tubercle and clavicle was ossified. Immediate intraoperative decompression of the subclavian vein and collateral vessels was seen in 11/13 procedures (85%). In the two patients with an occluded vein no change to the collateral flow was observed. Operative times ranged from 135 to 243 minutes with a mean of 187 minutes (+/- 30.8 minutes). Hospital stay ranged from 2-11 days with a median hospitalization of 4 days. Prolonged hospitalization occurred in two patients and was due to the management of other preexisting comorbidities. There were no surgical complications. There was no mortality. On postoperative venography, 4/13 (31%) extremities had persistent partial or complete occlusion of the subclavian vein. All of these extremities had partial or complete occlusion of the subclavian vein on preoperative venography. These patients underwent postoperative angioplasty and stent placement and received warfarin sodium and antiplatelet therapy for 3 months followed by daily aspirin (81mg). In 5/13 (38%) affected extremities the subclavian vein was patent postoperatively. These patients received anticoagulation for 3 months. In 4/13 (31%) unaffected extremities the subclavian vein was patent postoperatively. These patients did not receive anticoagulation. Follow-up was complete in all patients and ranged from 1- 20 months with a median of 12 months. At the time of follow up, the subclavian vein was open in all patients.Operative Steps
Positioning and Anesthesia
Phases of the operation
Phase I- Video-assisted thoracoscopic surgery (VATS) entry.
Phase II- Robotic rib dissection.
Phase III- VATS rib cutting.
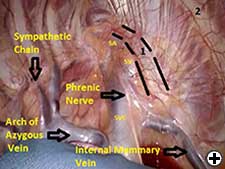
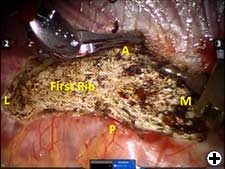
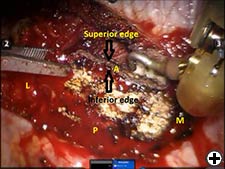
Phase IV- Robotic excision of the first rib.
Closure
Preference card
Tips and pitfalls
Results



