ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Robotic Thymectomy via Right Chest Approach
A right robotic thymectomy is described for the treatment of myasthenia gravis and thymoma. Early results are comparable to sternotomy and VATS approaches. A novel technique using ICG for contralateral phrenic nerve identification is also described.
Patient Selection
A minimally invasive approach to thymectomy for myasthenia gravis and small thymomas has been described and is attractive for multiple reasons. Prior to the availability of robotic approaches to thymectomy, we used a bilateral VATS approach with a neck incision for patients undergoing thymectomy for myasthenia gravis. Although we were able to complete the operation, the ability to reliably and reproducibly dissect the superior poles led us to use a neck incision for completeness, and the bilateral approach was necessary to definitively visualize the contralateral phrenic nerve. Because of these limitations we pursued a robotic approach to thymectomy, as the small compact space of the anterior mediastinum is ideally suited for a robotic approach given the 10x magnification and endowrist movements. Early adopters of robotic thymectomy have demonstrated some key advantages including shorter length of stay, quicker recovery, less pain and a comparable thymectomy relative to sternotomy [1,2] and VATS[3].
We have offered right-sided robotic thymectomy to all patients with myasthenia gravis. Early in our experience we restricted our patient selection to patients with thymomas less than 3 cm. However, with increasing experience we have liberalized the size cut off and now approach most anterior mediastinal masses using a right-sided robotic approach. The only exception is the anterior mediastinal mass that is predominantly left sided and localized in the AP window or juxtaposed to the course of the left phrenic nerve. We have not excluded patients based on BMI, ASA status, Osserman class or age. In fact, older patients, patients with higher BMI and those with worse ASA status may benefit from a minimally invasive approach compared to sternotomy.
Operative Steps
Before patients are brought to the OR suite, we turn the table around and use the foot as the head, with the addition of the head piece at this location. This allows the robot to dock unencumbered by the pedestal of the OR table.
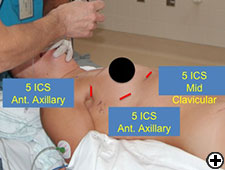
Patients are anesthetized in the supine position and a double lumen endotracheal tube is placed. Patients are positioned with the edge of the right side of the body resting at the edge of the table. This allows the right arm to be suspended below the plane of the body against the edge of the table (Figure 1). A long gel roll is then placed from the patient's hip to the shoulder, elevating the right hemithorax and posterior axillary line. Anesthesia personnel and equipment are positioned directly off the right shoulder and the robot is brought over the left shoulder. Prior to draping, the patient is placed in 5-10 degrees of reverse Trendelenburg to allow the diaphragm and mediastinum to fall away from the neck.
We begin by dropping the ipsilateral lung and preemptively placing 3 mL of bupivacaine 0.50% with epinephrine in the interspaces for each port site. Our first site is in the 5th interspace in the anterior axillary line, where we place a 12 mm trocar for the 12 mm 0 degree scope (Figure 1). We insufflate CO2 at a preset pressure of 6 - 8 mm Hg with a flow of 6 - 10 L/min. Our next port is placed in the 5th interspace in the mid clavicular line at least 10 cm away from the camera. This 8.5 mm port is used for robotic arm #1. The next port is placed higher in the anterior axillary line just posterior to the edge of the pectoralis major and at a level guided by the internal view so that the trajectory line into the chest will be inferior to the right internal mammary/vena caval junction. An 8.5 mm robotic port is used with robotic arm #2.
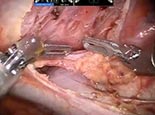
The robot is docked, with the boom coming in at an angle just over the left shoulder; it should line up directly with the camera port and the midpoint of the sternum. We typically begin with a 0 degree scope, a Cadière grasper in the right hand (arm #2) and a cautery hook in the left hand (arm #1). The mediastinal pleura is incised just anterior to the phrenic nerve, which is generally easy to visualize. We continue cephalad toward the internal mammary vein, transitioning anteriorly to the sternum, then dividing the pleura along the internal mammary vessels for several centimeters. At this point, the areolar tissue against the sternum is bluntly dissected and any visible vessels are cauterized. Similarly, we elevate the thymic tissue off the pericardium and retrace our steps cephalad until we identify the left brachiocephalic vein. (Video 1: Initial approach)
Once the vein is identified, the "L" hook is switched for the bipolar dissector then we will separate the thymus from the vein by tunneling anterior to the vein and underneath the thymus to facilitate identification of the left edge of the thymus. On the sternal side of the thymus, the dissection is carried across the mediastinum to expose the left internal mammary vessels. Often at this point we change to a 30 degree down scope and then, in succession, we dissect the right superior pole and the left superior pole. It is important to incise the investing fascia at this level and gently reduce each pole out of the neck. Using a hand over hand technique is helpful to gently deliver the superior poles. Defining the space between the poles is helpful (Video 2: Mobilization of the superior poles). The far side of the left pole is identified by placing the bipolar dissector anterior to the vein through the tunnel previously created. In this fashion, the Cadière forcep can gently retract that edge to begin reducing the pole. Once the upper poles are dissected the individual draining branches are clipped or controlled with cautery as appropriate (Video 3: Management of thymic veins).
Once both poles are mobilized, we open the left pleura widely from the origin of the left mammary vein to the diaphragm, following the vessels. It is helpful to reduce the tidal volume on the single left lung to limit the left lung from hindering the surgical view. Often we reduce it to 500 mL or less, depending on the patient's pulmonary status. Once the pleura is open, we identify the left phrenic nerve in one of three ways: 1) direct visualization by looking down intrapleurally with the 30 degree scope while retracting the pleura towards the right chest; 2) dissecting the left internal mammary complex to directly visualize the phrenic nerve origin; and/or 3) using indocyanine green (ICG) and the Firefly camera to fluoresce the pericardiophrenic artery and vein which run alongside the nerve. (Video 4: Identification of the contralateral phrenic nerve) Once we identify the nerve, we incise the pleura along its course. It is usually necessary to also free the thymus from the pericardium in the AP window at this point to facilitate phrenic nerve preservation in this region. To help with retraction and visualization, the thymus is reflected into the left chest (Video 5: Separation of contralateral phrenic nerve from thymus).
We use an Endo-bag™ (Covidien, Mansfield, MA) to remove the specimen. The sac is brought in via the mid clavicular incision and the specimen placed into the bag using the left robotic arm or standard thoracoscopic instruments. After the bag is closed a long string is left on the bag and clamped at the skin with a hemostat. The camera is then removed and the robot is undocked. A grasper is then brought in from the camera port anteriorly and the string is grasped and brought out the camera port. The camera incision is enlarged just enough to remove the specimen. We then hold the camera by hand, ensure hemostasis, perform rib blocks, and via the mid clavicular port insert a Blake drain that travels across the mediastinum into the left hemithorax. All sites are closed meticulously, taking care to close the deeper and more superficial tissues separately, as leakage of fluid can be a nuisance in the postoperative period and may require additional sutures on the ward or in the clinic after discharge.
Preference Card
It is important to include the OR staff and anesthesiology in the initial phase of planning for robotic thymectomy. Anesthesiology may prefer face forward proximity to the patient's airway for intraoperative double lumen endotracheal tube management. The OR staff will need to pre-plan OR equipment arrangement with anesthesia requirements in mind. In addition to the anesthesia machine, relevant equipment includes the robot, robotic console, OR bed, equipment boom, scope table, sterile back table, supply carts, monitors, scope warmer, and possibly bronchoscopy equipment.
- The OR bed is turned 180°, headpiece to foot, with pedestal end towards the feet to accommodate unencumbered docking of the robot as needed.
- Electrical cord management should ensure that surgeons have obstacle-free movement from one side of the patient to the other and also back and forth to the robot console, in a darkened room, as needed. Routine thoracic robot instruments include: Cadière, "L" hook cautery, curved bipolar dissector, and Hem-o-lock clips or other vascular clips.
- Plan for possible conversion to sternotomy by marking the sternal notch, sternal edges and xyphoid, especially during initial experiences with this approach.
- Develop a trained thoracic robotics staff.
- Adjunct OR team members initially should include an Intuitive trained staff member and vendor to ensure safe docking, routing and troubleshooting capabilities.
Tips and Pitfalls
- It does take some time to get used to appropriate docking and undocking of the robot in a timely fashion. In order to expedite the process it helps to have a nursing team that is consistent if possible.
- The ventilator tubing should be long so the anesthesiologists have plenty of room.
- The port sites should not be made too large or else the ports will slide excessively during the operation or allow CO2 to egress.
- The subcutaneous access to the ribs should be straight and not tunneled for any ports. If tunneling has occurred this should be redone as it will limit the motion and freedom of that robotic arm.
- The angle of the robot for docking is crucial, and should be brought into alignment with the camera port, anticipated center of the thymus and the left shoulder.
Results
We believe that complete thymectomy is the key to giving patients the best chance of being free from medical therapy for myasthenia gravis. The surgical borders of the robotic thymectomy are the same as those of open thymectomy via sternotomy: phrenic nerve to phrenic nerve, base of diaphragm to superior poles. Consistently mobilizing both complete superior poles robotically took time to master. Early in our experience, we started with a 2 cm collar incision to mobilize the superior poles in the neck. This allowed us to understand the anatomy from the chest since it was easy to delineate the right and left sides. As we became more experienced, we made the collar incision after removal of the thymus to determine if we left thymus behind at the poles. At the present time, we use a collar incision only when we have not reached the end of the superior pole or at frozen section have elements of thymus at the tip of the resected upper poles.
A consistent and reproducible method for identification of the contralateral phrenic nerve is one area that needs continued study and validation. If the patient is thin, the identification is straightforward. However, in the obese individual, identification can be challenging and adds time during the operation. On the Si version of the robot, the fluorescence imaging system (Firefly) has the potential to more definitively identifying the nerve by fluorescing the pericardiophrenic artery and vein that run parallel to it.
Since transitioning to right robotic thymectomy, we have performed virtually all of our thymectomies in this fashion. In 2010 we reviewed a small subset of our thymectomies comparing those done minimally invasively (VATS and robotic) to sternotomy.[3] This showed a significant reduction in length of stay with similar operative outcomes such as operative time and blood loss. Since that report, we have been able to reduce our length of stay by an additional day with most patients being discharged on the first postoperative day and returning to usual activities within the first two weeks postoperatively off narcotics. With experience the operative time has been reduced.
Disclosure
Drs. Louie and Farivar disclose a relationship with Intuitive Surgical. Both receive honoraria as speakers and surgical proctors.
References
- Goldstein SD, Yang SC. Assessment of robotic thymectomy using the Myasthenia Gravis Foundation of America Guidelines. Ann Thorac Surg 2010;89(4):1080–6.
- Cakar F, Werner P, Augustin F, Schmid T, Wolf-Magele A, Sieb M, et al. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur J Cardio-Thorac Surg 2007;31(3):501–5.
- Youssef SJ, Louie BE, Farivar AS, Blitz M, Aye RW, Vallières E. Comparison of open and minimally invasive thymectomies at a single institution. Am J Surg 2010;199(5):589–93.