ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Minimally Invasive Cardiac Surgery Through Periareolar Approach
Marin Cuartas M, Saldaña LD, Quintero AA, Jaramillo JS, Rendon JC. Minimally Invasive Cardiac Surgery Through Periareolar Approach. July 2018. doi:10.25373/ctsnet.6815693.
Introduction
Since the early 1990s, minimally invasive cardiac surgery (MICS) has evolved worldwide. It started with mitral valve procedures and then gradually expanded toward other valve procedures, coronary artery bypass grafting, and various types of simple congenital heart operations. There has been a growing interest for MICS, given the association of this technique with decreased pain, shorter hospital stay, accelerated recuperation and cost effectiveness (1, 2). Recently, better aesthetic results have also been sought without compromising the size of the surgical field and the exposure of anatomical structures. In response to this, a new type of approach known as periareolar incision has been developed over the last years (3 - 5). The aim of this study was to describe the authors’ experience and to evaluate the feasibility of MICS through a periareolar approach.
Materials and Methods
This retrospective observational study describes the postoperative outcomes in patients undergoing multiple cardiac surgical procedures through periareolar approach from January 2015 to December 2016 at the authors’ institution. An assessment of preoperative (demographic) and operative variables such as surgical diagnosis and procedure was carried out, as shown in Table 1. Procedural technical aspects such as cross-clamping and perfusion times were appraised, together with postoperative variables such as thoracic drainage during the first 24 hours, intensive care unit (ICU) length of stay (LOS), total hospital LOS, and postoperative complications, as shown in Table 2. Echocardiographic control was performed just before hospital discharge in all patients who underwent mitral valve repair, as shown in Table 3. In an exploratory manner, survival follow-up was performed by means of telephone contact and periodical medical control until the end of the study. All results were reported as absolute values, ranges, and percentages. The development of the study was approved by the ethics committee of the CardioVID heart center (Medellìn, Colombia). Informed consent about the procedure was signed by the patient and the surgeon before each surgery.
Table 1. Demographic and operative variables.
| Total periareolar MICS, n | 48 |
| Female sex, n (%) | 40 (83) |
| Mean age, years ± SD (range) | 48.35 ± 14.35 (14 - 73) |
| Atrial septal defect/sinus venosus closure, n (%) | 19 (39.6) |
| Mitral valve repair, n (%) | 11 (23) |
| Isolated mitral valve replacement, n (%) | 12 (25) |
| Mitral valve replacement combined with other procedures, n (%) | 3 (6.25) |
| Cardiac tumor resections, n (%) | 2 (4.2) |
| Off-pump atrial perforation repair, n (%) | 1 (2.1) |
MICS, minimally invasive cardiac surgery; SD, standard deviation
Table 2. Procedural technical aspects and postoperative variables.
| Mean aortic cross-clamping time, minutes ± SD (range) | 69.29 ± 28.83 (0 - 146) |
| Mean perfusion time, minutes ± SD (range) | 113.08 ± 47.30 (0 - 261) |
| First day median thoracic drainage, ml (range) | 255 (0 - 2000) |
| Median ICU LOS, hours (range) | 26 (0 - 120) |
| Median hospital LOS, days (range) | 5 (2 - 17) |
| Postoperative complications | |
| Coagulated hemothorax, n (%) | 2 (4.2) |
| Atrioventricular block, n (%) | 1 (2.1) |
| Breast hematoma, n (%) | 1 (2.1) |
| Wound infection, n (%) | 0 (0) |
ICU, intensive care unit; LOS, length of stay; SD, standard deviation
Table 3. Mitral valve repair through periareolar approach (n = 11).
| Female, n (%) | 6 (54.5) |
| Male, n (%) | 5 (45.4) |
| Mean age, years ± SD (range) | 53.5 ± 16.2 (13 - 72) |
| Mean body mass index ± SD (range) | 24.6 ± 4.03 (18 - 33) |
| Severe mitral regurgitation, n (%) | 7 (64) |
| Degenerative disease, n (%) | 10 (91) |
| Rheumatic disease, n (%) | 1 (9) |
| Neochordae, n (%) | 7 (63.6) |
| Triangular leaflet resection, n (%) | 2 (18) |
Surgical Technique
All patients were intubated with a double lumen tube for single lung ventilation in order to isolate the lung ipsilateral to the surgery site. A cloth roll was inserted behind the patient between both scapulae obtaining a 15- to 30-degree position. It is mandatory to place external defibrillation pads.
Femoral incision is performed for dissection of the femoral vessels. Purse string sutures with 4-0 or 5-0 polypropylene were placed in each vessel. Cannulation of the femoral vessels was done with semi-Seldinger technique under transesophageal echocardiographic guidance. The arterial cannulation was performed with an EOPA® Cannula (18-20 Fr) and the femoral vein was cannulated with a Bio-Medicus™ cannula (25 Fr). For patients undergoing atrial septal defect (ASD) closure, the superior vena cava was also cannulated with a Bio-Medicus™ cannula (15-17 Fr) through a percutaneous jugular puncture. A 180 degree incision following the edge of the nipple-areola complex was performed. The glandular tissue was then dissected with cautery until the pectoralis major muscle was reached. Afterwards, an incision through the intercostal muscles was performed at the fourth intercostal space. A soft tissue retractor (Alexis® Retractor, Applied Medical) was inserted and a thoracic retractor was then placed over it. The pericardial fat was removed and the pericardium was opened 3 cm above the phrenic nerve. Then, two small skin incisions (3 - 4 mm) were performed in order to introduce four pericardium separation sutures for better exposition. After cardiopulmonary bypass (CPB) was initiated, superior and inferior vena cavae snares (silk or umbilical tapes) were placed through the periareolar incision but not secured. A 1 cm skin incision was then performed for cardioplegia/vent needle insertion into the ascending aorta. Thereafter, another 1 cm skin incision was performed on the intersection of the second intercostal space with the anterior axillary line for insertion of the Chitwood aortic clamp into the mediastinum. Thereafter, ascending aorta was cross-clamped and cold del Nido cardioplegia was delivered. Once the heart had arrested, the femoral venous cannula was repositioned and the snares around the inferior and superior cavae were secured.
For mitral valve surgery, the left atrium was incised and the atrial retractor was inserted. Mitral valve replacement or repair was performed with the conventional MICS technique. The left atrium was sutured with 4-0 polypropylene running suture. Intraoperative transesophageal echocardiography was always performed in order to ensure accurate repair of the mitral valve.
For ASD and sinus venosus closure, a transverse incision through the right atrium was performed and separation sutures on the atrium wall were placed for a better exposure. After identification of the septal defect, all ASD and sinus venosus were corrected with bovine pericardial patch sutured with 4-0 polypropylene running suture. Thereafter, the right atrium was closed with a 5-0 polypropylene running suture.
After closure of the atrium, cardioplegia needle was connected to an aspiration line for deaeration and the aortic clamp was released. Once CPB was finished, heparin was reversed, hemostasis was revised, and cannulations were removed. The femoral artery was sutured, ie, reconstructed, and the femoral incision was closed in layers. One ventricular epicardial electrode was attached to the heart and then the pericardium was closed with three interrupted polyester sutures. Blake or silicone drains were left inside the pericardium and pleural space. The periareolar incision was closed in layers with absorbable suture, beginning with the muscular layer and continuing with the glandular tissue. The nipple-areola complex was sutured subdermally with poliglecaprone suture (Monocryl®, Ethicon).
Results
A total of 48 patients underwent MICS through a periareolar approach. The mean age of the patients was 48.35 ± 14.35 years (range, 14 - 73) and 40 of 48 (83%) of the patients were women. The authors performed 19 (39.6%) ASD/sinus venosus closures, 11 (23%) mitral valve repairs, 12 (25%) isolated mitral valve replacement, 3 (6.25%) mitral valve replacements combined with other procedures, 2 (4.2%) cardiac tumor resections, and 1 (2.1%) off-pump atrial perforation repair. All mitral valve repairs were performed with ring annuloplasty, seven patients required neochordae, and two patients required triangular leaflet resection (see Table 4). Mean times for aortic cross-clamping and perfusion were 87.91 ± 19.36 minutes (range, 59 - 134) and 144.45 ± 42.13 minutes (range, 71 - 201), respectively. There was no need for conversion to minithoracotomy or sternotomy. The median thoracic drainage was 255 ml during the first 24 hours (0 - 2000 ml). Only one patient presented significant bleeding (2000 ml) but no reintervention was required to control it. Mean ICU and mean total hospital LOS were 26 hours (range, 0 - 120) and five days (range, 2 - 17), respectively. Survival rate up to February 2018 is 98%, due to one patient undergoing mitral valve replacement who died within the first 30 days postoperatively from malignant hyperthermia.
Table 4. Echocardiographic results at hospital discharge after mitral valve repair through periareolar approach.
| MR, any degree | 11 |
| No regurgitation | 9 |
| Mild (+1) | 2 |
| Moderate (+2) | 0 |
| Severe (+3) | 0 |
MR, Mitral Regurgitation
Complications were present in four cases: two patients presented coagulated hemothorax, one patient presented atrioventricular block, and one patient presented a breast hematoma, which resolved after surgical drainage. Echocardiographic control at hospital discharge revealed accurate mitral valve repair in all cases. Only two patients presented mild mitral regurgitation (MR), as shown in Table 3. All of the patients were very comfortable with the cosmetic results and no concerns were reported about loss of sensitivity.
Discussion
MICS has evolved since the first cases performed by Navia and Cosgrove (6) and Cohn et al (7). Along with the evolution of surgical and perfusion techniques, there has been a technological development of instruments and cannulas that facilitates this type of procedure (8 - 11). MICS has gained interest during the past two decades not only for surgeons but also for patients, given the fairly well documented advantages such as shorter ICU and hospital LOS, as well as shorter recovery times, less bleeding and transfusion requirements, and better cosmetic results. After an accurate learning curve, these procedures are as safe as the conventional approach (11 - 14).
Different types of incision have been described for MICS (1, 2), depending of the kind of surgery that is going to be performed. Alternative approaches are continuously developed in order to reduce the size of the incisions, thus improving aesthetic results, without compromising a safe and accurate surgical technique (Figure 1). The periareolar incision is described for many plastic surgery procedures. However, only a few reports have described this approach in the context of cardiac surgery (3, 15, 16).
The main consideration of the periareolar approach is the adequate selection of the patient. For this reason, in order to properly choose the patient, besides the physical examination of the breasts, different considerations should be taken into account such as age, gender, weight, type of surgery, cannulation, thoracic configuration, size of the areola, and previous surgeries or irradiation of the breast.
Periareolar surgery can be performed in both men and women without any difference related to the results. The areola should have a diameter of at least 3 cm. In those cases where the areola has a diameter of less than 3 cm, a lateral lineal incision of 1 cm can be made to enlarge the opening of the incision. The periareolar incision should not extend over 180 degrees around the areola in order to guarantee the preservation of the vascular and nerve supply of the nipple-areola complex and prevent necrosis and loss of sensitivity (16). Patients with large anteroposterior diameters are better candidates since those with small diameters often do not have enough space to insert the valves of the thoracic retractor comfortably.

Figure 2. Flattening of the breast after accurate positioning on the surgical table of an obese female patient.
This is, however, a clinical-based appreciation because the authors do not routinely perform a computed tomography scan before surgery. In obese female patients, the breast and the axillary fat could be profuse. Nevertheless, when the patient is positioned on the surgical table, the breast flattens around the areolar region allowing a normal periareolar incision or even making the procedure much easier for the surgeon than in thin patients, as seen in Figure 2.
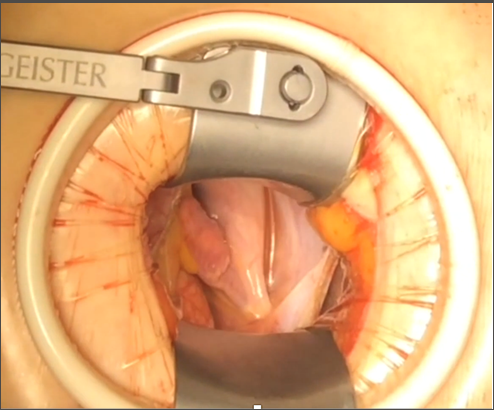
Figure 3. Adequate visualization of the cardiac structures through the periareolar incision, thus allowing a depurate surgical technique.
It has to be clearly remarked, that benefit of the periareolar incision could be even more technical than aesthetic according to the type of surgery being performed (Figure 3). This approach is optimal for correction of ASD since it gives the surgeon a more anterior visualization, which is very helpful for the patch suture. Venous sinus type ASD especially benefits from this technique. Considerations concerning cannulation are the same as for all other MICS techniques. Special attention should be paid to the diameter and quality (thrombosis or calcification) of femoral vessels. Based on the authors' experience, breast implants are not a contraindication for the periareolar approach. The breast prostheses can be explanted and kept within saline solution with diluted antibiotics (first generation cephalosporins) during the procedure. Once the procedure has been finished and the pectoralis major and capsule are closed, the prosthesis is reimplanted with help of a plastic surgeon. The authors have performed five MICS cases through periareolar incision for mitral valve surgery in patients with breast implants without any complications. Patients with personal history of breast irradiation or surgery for cancer as well as reconstructive breast surgery (mastopexy) are not good candidates for the periareolar approach (Table 5), because previous vascular compromise is unknown and therefore the areola-nipple complex could be at risk of necrosis (17).
Table 5. General considerations for a periareolar approach.
| Gender | no concern about sex, possible for both male and female patients |
| Areolar diameter | ≥3 cm |
| Incision | should not extend over 180 degrees (Figure 4) |
| Obese patients | breast flattens around the areolar region, allowing a normal periareolar incision after patient is positioned on the surgical table |
| ASD/sinus venosus | The procedure especially benefits from this technique. It gives the surgeon a better visualization for patch closure. |
| Breast implants | Breast implants are not a contraindication. The breast prostheses can be kept within saline solution with diluted antibiotics during the procedure. |
| Contraindications | history of breast irradiation or surgery for cancer, history of reconstructive breast surgery (mastopexy) |
In another not yet published study that is still running at the author’s heart center, they have documented the aortic cross-clamp and CBP times for mitral valve repair through right minithoracotomy. The authors have not found any statistically significant differences for cross-clamping (P = 0.07) and CBP (P = 0.75) times among patients undergoing mitral valve repair through right minithoracotomy and patients operated through periareolar incision (Table 6). However, patients selected for the standard right minithoracotomy approach presented more complex mitral valve disease and therefore more challenging mitral repairs, which has also been the reason to exclude those patients for the periareolar approach. Moreover, the learning curve for the periareolar approach is still growing and therefore this technique seems to be promising also for complex mitral pathologies. In this series, one patient died from a noncardiac and/or nonsurgery-related cause. In view of the fact that the death was caused by an anesthetic adverse event, the authors consider that the mortality related to the surgical technique per se is 0% for this series.
Table 6. Time comparison between periareolar approach and right minithoracotomy for mitral valve repair
| Periareolar (n = 48) | Thoracotomy (n = 114) | P - value | |
| Aortic cross-clamping time, min | |||
| Mean ± SD | 87.91 ± 19.36 | 104.56 ± 29.95 | 0.07 |
| Median (range) | 85 (59 - 134) | 98 (34 - 228) | |
| Perfusion time, min | |||
| Mean ± SD | 144.45 ± 42.13 | 148.64 ± 41.73 | 0.75 |
| Median (range) | 153 (71 - 201) | 139 (78 - 293) |
Min, minutes; SD, standard deviation
This study is limited by the small sample size. Additionally, it suffers from the inherent problems of a retrospective research. Nevertheless, the authors believe that they have a wide experience performing MICS through periareolar approach and therefore consider that their appreciations may be useful for others who are interested in the technique. The doors are left open for prospective studies with long-term follow-up and a larger number of patients.
Conclusions
After adequate patient selection, periareolar MICS is a feasible and safe approach which allows an accurate surgical technique, without increasing surgery times or complications. ASD and sinus venosus closure especially benefit from this technique because of a better visualization. Aesthetical results are better when compared with other incision types. Postoperative results are comparable to other MICS techniques.
References
- Marin Cuartas M, Javadikasgari H, Pfannmueller B, et al. Mitral valve repair: robotic and other minimally invasive approaches. Prog Cardiovasc Dis. 2017;60(3):394-404.
- Ritwick B, Chaudhuri K, Crouch G, Edwards JR, Worthington M, Stuklis RG. Minimally invasive mitral valve procedures: the current state. Minim Invasive Surg. 2013;2013:679276.
- Bozso SJ, Grant A, Iglesias I, Chu MWA. Minimally invasive periareolar approach to unroofed coronary sinus atrial septal defect repair. Ann Thorac Surg. 2016;102(3):e223-e225.
- Poffo R, Pope RB, Toschi AP, Mokross CA. Video-assisted minimally invasive mitral valve repair: periareolar approach [in Portuguese]. Rev Bras Cir Cardiovasc. 2009;24(3):425-427.
- Poffo R, Pope RB, Selbach RA, et al. Video-assisted cardiac surgery: results from a pioneer project in Brazil [in Portuguese]. Rev Bras Cir Cardiovasc. 2009;24(3):318-326.
- Navia JL, Cosgrove DM III. Minimally invasive mitral valve operations. Ann Thorac Surg. 1996;62(5):1542-1544.
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg. 1997;226(4):421-428.
- Soltesz EG, Cohn LH. Minimally invasive valve surgery. Cardiol Rev. 2007;15(3):109-115.
- Schmitto JD, Mokashi SA, Cohn LH. Minimally invasive valve surgery. J Am Coll Cardiol. 2010;56(6):455-462.
- Kronzon I, Matros TG. Intraoperative echocardiography in minimally invasive cardiac surgery and novel cardiovascular surgical techniques. Am Heart Hosp J. 2004;2(4):198-204.
- Chitwood WR Jr, Elbeery JR, Moran JF. Minimally invasive mitral valve repair using transthoracic aortic occlusion. Ann Thorac Surg. 1997;63(5):1477-1479.
- Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg. 2008;34(4):760-765.
- Mohr FW, Falk V, Diegeler A, Walther T, van Son JA, Autschbach R. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg. 1998;115(3):567-576.
- Davierwala PM, Seeburger J, Pfannmueller B, et al. Minimally invasive mitral valve surgery: "the Leipzig experience". Ann Cardiothorac Surg. 2013;2(6):744-750.
- Dieberg G, Smart NA, King N. Minimally invasive cardiac surgery: a systematic review and meta-analysis. Int J Cardiol. 2016;223:554-560.
- Abreu LM , Alves MLM, Honório J, et al. Video-assisted surgery via periareolar, mitral valve replacement and papillary muscle relocation with neochordae of PTFE. J Cardiothorac Surg. 2013;8(Suppl 1):P144.
- van Deventer PV, Graewe FR. Enhancing pedicle safety in mastopexy and breast reduction procedures: the posteroinferomedial pedicle, retaining the medial vertical ligament of Würinger. Plast Reconstr Surg. 2010;126(3):786-793.





Comments