Introduction
The term ‘diaphragmatic eventration’ is used in common practice to describe a condition of relaxation of the diaphragmatic dome. It may present at birth as a congenital condition due to a defect of diaphragmatic development or in a later stage of life as an acquired condition (‘acquired diaphragmatic paralysis’ or ‘acquired diaphragmatic elevation’).
We present a case of a 37-year-old African American female with an unremarkable past medical history, who denied any history of trauma or previous surgery. She presented to the emergency department with a 6-month history of worsening exertional dyspnea. She also had been experiencing worsening abdominal distension and constipation requiring use of laxatives.
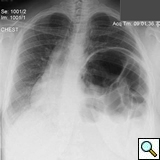
On physical exam she was obese (BMI 34) but in good general condition. There were no abnormalities on the thoracic and abdominal examination other than reduced breath sound at the left lung base. A chest x-ray showed substantial elevation of the left hemidiaphragm (Figure 1). A chest CT showed a massively distended left colon under the left diaphragm (Figure 2). A “sniff test” (fluoroscopic assessment of diaphragmatic function) showed paradoxical motion of the elevated hemidiaphragm.
Because of the extreme diaphragmatic elevation an open approach through the chest was selected for correction of the abnormality. After preoperative bowel preparation, the patient was taken to the operating room and a muscle-sparing left antero-lateral thoracotomy was performed in the 5th intercostal space. With this approach the free edge of the latissimus dorsi is mobilized posteriorly and the serratus anterior is freed up at the level of its anterior insertion on the chest wall, allowing easier access to the anterior portion of the diaphragmatic leaflet. The diaphragm appeared morphologically normal but relaxed (Figure 3).
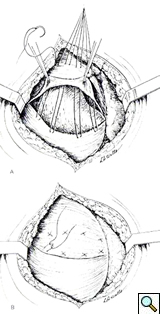
The left dome was opened anteriorly and towards the periphery following the line of insertion into the chest wall, in order to avoid injury to the major branches of the phrenic nerve. The transverse colon and the spleen were displaced inferiorly through the opened diaphragm. The colon was massively distended and was carefully reduced into the abdominal cavity. The redundancy of diaphragmatic dome was such that the posterior edge had to be trimmed removing an elliptical segment of muscle of 14 x 8 cm in size. The diaphragm was reconstructed overlapping the two edges of the opened muscle with interrupted, size O, nonabsorbable U-shaped sutures in a “double-breasted” reconstruction technique, reinforced by a second running layer (Figure 4).
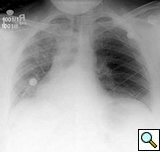
The patient’s postoperative course was uncomplicated and she was discharged on postoperative day 4. She was seen again in the outpatient clinic approximately a week later and a chest x-ray demonstrated a normal left diaphragm contour (Figure 5).
She had no complaints. Her exertional dyspnea was resolved, but she was still experiencing some constipation that eventually improved in the following weeks with the help of stool softeners. At time of her last follow-up visit, twelve months later, she remained asymptomatic.
Discussion:
According to most authors, ‘true’ eventration is always a congenital condition due to a defect of development of one portion or the entire central part of the diaphragm [1-4] and is characterized by a membranous appearance of the muscle with a marked decrease in muscle fibers compared to the normal muscle [4, 5]. This condition is present since birth and, if severe, can manifest in the neonatal period with severe cardio-respiratory symptoms secondary to hypoplasia of the lung on the affected site. On the other hand, ‘acquired paralysis’ or ‘acquired elevation’ is a condition of relaxation of the diaphragm that occurs in older children and adults and that is thought to be caused by an acquired complete or incomplete paralysis of a diaphragmatic leaf and not by a congenital defect of diaphragmatic development [3, 4]. In the case of ‘true’ eventration there is a congenital defect in the musculature of the diaphragm, whereas in the case of ‘acquired paralysis’ there is a loss of contractility of the muscle that leads to progressive muscular atrophy and distension of the dome. It can be secondary to trauma, infection such as polio, herpes zoster, diphtheria, or influenza [3], neoplastic diseases, or autoimmune pathologies directly involving the diaphragm or the phrenic nerve. In particular, the ‘idiopathic’ form, which more frequently affects adults, is considered the result of a subclinical viral infection and presents more commonly with unilateral involvement. Surgical correction of ‘acquired paralysis’ is indicated in any case where there is evidence of respiratory compromise [3, 4] without resolution of the condition over a short period of observation (3 to 6 months) [4]. Exertional dyspnea severe enough to impair simple daily activity is the most common indication for surgery [3, 4]. Before proceeding with surgical correction, it is crucial to rule out other possible causes of dyspnea [3].
Studies comparing respiratory function tests before and after correction of the diaphragm showed significant improvement in all lung volumes, DLCO, and resting pO2 in a 5 to 12 year follow-up [5, 6]. The real difference between ‘true’ eventration and the acquired form is that the latter may resolve spontaneously [4, 7], whereas the congenital form does not improve with time and is actually expected to become symptomatic at a very early stage of life due to impairment of the development of the underlying lung[8, 9].
The repair is most easily performed through the chest, with a lateral thoracotomy at the 7th or 8th intercostal space [4]. The strategy of surgical repair has to be adapted to each specific case. The approach through the chest makes the correction of the diaphragm technically easier. It provides exposure over the convexity of the diaphragmatic dome and allows a more precise estimation of the tension that is needed to obtain an optimal correction. The goal of surgical correction is to place the diaphragmatic leaflet in a position of maximum inspiration which relieves compression on the lung parenchyma and allows its re-expansion [4, 10, 11]. The repair of the diaphragm is accomplished by plication of the diaphragm or by imbrication. Imbrication is performed by opening the diaphragmatic dome and overlapping the two edges of the muscle (Figure 4). When the diaphragm is substantially elevated, resection of a portion of diaphragm is required, followed by reconstruction with a double breasted suturing technique.
Thoracosopic and video-assisted approaches have also been used successfully for the correction of symptomatic diaphragmatic paralysis [10, 11, 12]. These techniques have the obvious advantage of facilitating postoperative recovery [3], but require advanced thoracoscopic expertise and should be used cautiously in cases of extreme elevation of the diaphragmatic dome [13]. The technique described by Mouroux and colleagues (1996) is carried out by VATS approach applying two ports, one for the thoracoscope and one for a working port. Using an instrument inserted through the working port, the diaphragmatic leaf is invaginated creating a transverse fold that can be sutured with nonabsorbable material through a minithoracotomy performed at the level of the ninth intercostals space. This thoracoscopic technique has been modified through the years to a pure thoracoscopic approach which is based on the use of head up position and CO2 insufflation to allow suturing of the diaphragm without need for a minithoracotomy. [14, 15, 16].
In summary, diaphragmatic paralysis is a condition with multiple possible etiologies, which not uncommonly may present to the general or thoracic surgeon. In adults, careful evaluation is required before proceeding with surgical correction. It is imperative to rule out other possible causes that may be responsible for the respiratory symptoms and consider conservative treatment measures, such as respiratory rehabilitation and weight loss, before proceeding with surgical repair. Surgical correction is indicated in cases of substantial diaphragmatic elevation associated with respiratory symptoms impairing routine daily activities. Open thoracotomy is the preferred approach but thoracoscopic techniques may be utilized with very good results in selected cases.
References
- Chin EF, Lynn RB. Surgery of the eventration of the diaphragm. J Thorac Surg 1956;32:6.
- Simoneau G, Sartene R, Girard P et al. Les disfunctions diaphragmatiques. Revue du Practicien 1983;33:2155.
- Frechette E, Cloutier R, Deslauriers J. Congenital eventration and acquired elevation of the diaphragm. In: Pearson FG, Cooper JD, Deslauriers J, et al., ed. Thoracic Surgery, II ed. Philadelphia: Churchill Livingstone: 2002:1537-1549.
- Shields, TW. Diaphragmatic function, diaphragmatic paralysis, and eventration of the diaphragm. In: Shields TW, LoCicero III J, Ponn R, Rusch VW: General Thoracic Surgery, VI ed. Philadelphia: Lippincott Williams & Wilkins: 2005:740-45.
- Wright CD, Williams JG, Ogilvie CM, Donnelly RJ. Results of diaphragmatic plication for unilateral diaphragmatic paralysis. J Thorac Cardiovasc Surg 1985;90:195-98.
- Graham DR, Kaplan D, Evans CC, Hind CR, Donnelly RJ. Diaphragmatic plication for unilateral diaphragmatic paralysis: a 10-year experience. Ann Thorac Surg 1990;49:248-51.
- Douglass BE, Clagett OT. The prognosis in idiopathic diaphragmatic paralysis. Chest 1960;37:294-97.
- Gaultier C. Development post-natal du poumon humain: ses rapports avec la function pulmonair et la pathologie respiratoire. Ann Pediatr 1976;23:447.
- Reviloony Y, Fekete CN: Eventration diaphragmatique chez l’enfant. Etude de trent-six cas de 1951 a 1980. Ann Chir Thorac Cardivasc 1982;36;71.
- Hwang Z, Shin JS, Cho YH, Sun K, Lee IS. A simple technique for the thoracoscopic plication of the diaphragm. Chest 2003;124:376-78.
- Mouroux J, Padovani B, Poirer NC, et al. Technique for the repair of diaphragmatic eventration. Ann Thorac Surg 1996;62:905-907.
- Knight SR, Clarke CP: VATS plication of the diaphragmatic eventration. Ann Thorac Cardiovasc Surg 1998;4:240-43.
- Hazelrigg SR: Commentary: Knight SR, Clarke CP: The diaphragm. In Yim APC, Hazelrigg SR, Izzat MD, et al. (eds): Minimal Access Cardiothoracic Surgery. Philadelphia, WB Saunders: 2000:301.
- Moon SW, Wang YP, Kim YW, Shim SB, Jin W. Thoracoscopic plication of diaphragmatic eventration using endostaplers. Ann Thorac Surg 2000;70:299-300.
- Leo F, Venissac N, Morales F, Rodriguez A, Mouroux J. Plication for diaphramatic eventration. Chest 2004;125:1170-71.
- Kim DH, Hwang JJ, Kim KD. Thoracoscopic diaphragmatic plication using three 5 mm ports. Interact CardioVasc Thorac Surg 2007;6:280-81.