ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Aortic Implantation for Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery
Patient Selection
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital anomaly that is one of the most common causes of myocardial ischemia and infarction in children. If untreated, it results in a mortality rate of up to 90% within the first year of life. In the current era, establishment of a two coronary artery system is considered the goal for repair of ALCAPA. There are several surgical techniques that have been developed to achieve this goal but currently aortic implantation appears to be becoming the procedure of choice at most institutions. The establishment of a two coronary artery system has been shown to result in complete recovery from myocardial dysfunction despite the severe nature of the preoperative left ventricular failure. The experience of many congenital heart surgeons with the arterial switch operation has led to the current popularity of aortic implantation as the procedure of choice. In this review, we analyze our surgical strategy including the conduct of cardiopulmonary bypass, cardioplegia administration, operative technique, and postoperative inotropic support. All patients with a diagnosis of ALCAPA should be operated on with a goal of providing a two-coronary system, usually within days of making the diagnosis. No matter how severe the ventricular dysfunction appears, we would currently recommend aortic implantation as the primary therapy, rather than proceeding, even in severe cases, to cardiac transplantation.
Operative Steps
Anesthesia/Monitoring/Positioning
A median sternotomy incision is the approach for this technique which requires the use of cardiopulmonary bypass. Transesophageal echocardiography is useful to assess the postoperative patency of the reimplanted coronary artery and assess the postoperative function and degree of mitral insufficiency. Central venous access is obtained with a double-lumen left subclavian central line. Radial arterial monitoring is used. We have administered IV dexamethasone (1 mg/kg IV) and aprotinin to all cardiopulmonary bypass patients. The right neck should be prepped and draped into the field in case postoperative ECMO/cardiopulmonary bypass is required.
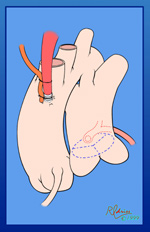
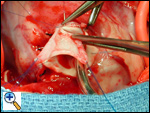
Aortic Cannulation
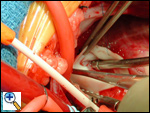
 The ascending aorta should be cannulated very close to the innominate artery to provide a long length of ascending aorta for the cardioplegia administration site and for the orifice that is to be created on the side of the aorta for the aortic implantation. The schematic view shows the typical location of the anomalous left coronary artery from the posterior sinus of the main pulmonary artery. The operative photographs are from the surgeon’s view. The aorta has been cannulated quite high. Rumel tourniquets have been passed around the right and left pulmonary arteries and have been employed once bypass has started. This helps to maintain a closed chamber in the proximal main pulmonary artery and increase the pressure in the left coronary artery system, assisting in perfusing the portion of the myocardium supplied by the anomalous left coronary artery. We use bicaval cannulation to divert all “warm” blood from the heart during the cross-clamp period. A vent is placed in the right superior pulmonary vein and passes through the left atrium into the left ventricle to decompress the left ventricle during the procedure. The patient is cooled to 28ºC.
The ascending aorta should be cannulated very close to the innominate artery to provide a long length of ascending aorta for the cardioplegia administration site and for the orifice that is to be created on the side of the aorta for the aortic implantation. The schematic view shows the typical location of the anomalous left coronary artery from the posterior sinus of the main pulmonary artery. The operative photographs are from the surgeon’s view. The aorta has been cannulated quite high. Rumel tourniquets have been passed around the right and left pulmonary arteries and have been employed once bypass has started. This helps to maintain a closed chamber in the proximal main pulmonary artery and increase the pressure in the left coronary artery system, assisting in perfusing the portion of the myocardium supplied by the anomalous left coronary artery. We use bicaval cannulation to divert all “warm” blood from the heart during the cross-clamp period. A vent is placed in the right superior pulmonary vein and passes through the left atrium into the left ventricle to decompress the left ventricle during the procedure. The patient is cooled to 28ºC.
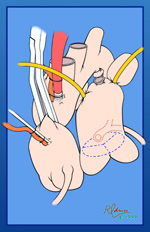
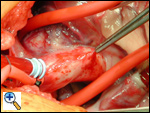
Cardioplegia Administration
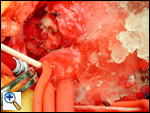 The aortic cross clamp has been placed and a cardioplegia needle is being used to infuse cardioplegia into the proximal ascending aorta. The snares on the right and left pulmonary arteries continue to create a closed chamber in the main pulmonary artery, completed by the closed pulmonary valve. This allows increased pressure in the anomalous left coronary system and should increase the perfusion of the myocardium supplied by that coronary. In the photograph, the cardioplegia is being administered, the right and left pulmonary arteries are snared, and ice slush has been delivered to cool and protect the myocardium.
The aortic cross clamp has been placed and a cardioplegia needle is being used to infuse cardioplegia into the proximal ascending aorta. The snares on the right and left pulmonary arteries continue to create a closed chamber in the main pulmonary artery, completed by the closed pulmonary valve. This allows increased pressure in the anomalous left coronary system and should increase the perfusion of the myocardium supplied by that coronary. In the photograph, the cardioplegia is being administered, the right and left pulmonary arteries are snared, and ice slush has been delivered to cool and protect the myocardium.
Pulmonary Artery Transection/Coronary Button Development
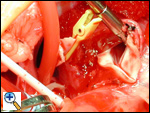
After the first dose of cardioplegia, the main pulmonary artery is transected after fully mobilizing the right and left pulmonary arteries and ligating and dividing the ligamentum arteriosum or patent ductus arteriosus. The coronary artery button is developed in a fashion similar to developing a button for a coronary transfer in the arterial switch operation. The photograph shows the partially transected main pulmonary artery. The cardiotomy sucker is immediately adjacent to the orifice of the anomalous left coronary artery.
The left main coronary artery has been mobilized off of the epicardium. After completing this dissection, a second dose of cardioplegia is administered and the orifice of the anomalous left coronary artery is occluded with a small plastic vascular clip. The cardiotomy sucker is now in the pulmonary valve orifice. This allows a second dose of cardioplegia to be delivered at approximately 20 minutes after the first cardioplegia dose. The second dose of cardioplegia is usually sufficient for the remaining portion of the operation.
The pulmonary valve cusp with the excised sinus where the anomalous left coronary arose will be reconstructed later with pericardium.
Coronary Button Implantation
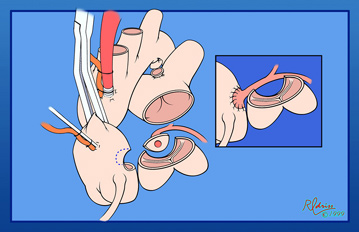
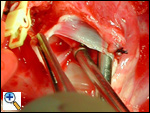
After the second dose of cardioplegia, an opening is created in the left posterolateral wall of the ascending aorta for implantation of the anomalous left coronary artery button. Care must be taken when creating this orifice that the aortic valve is not injured or the orifice of the right coronary artery. This orifice is smaller than the coronary button by a factor of 30% to 40%. The large button of coronary artery can then act as a “conduit” for elongation of the left coronary artery.

The photograph shows a forceps holding the coronary artery button with a significant amount of pulmonary artery attached to the button. Inferiorly the opening in the aorta has been created with a small flap of the aortic wall being pulled anteriorly. This flap of aortic wall can also be used to augment and extend the anastomosis if the coronary button appears tight.
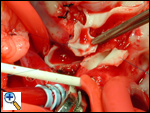
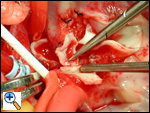
The anomalous left coronary artery-to-aorta anastomosis is started inferiorly and the suturing commences in a "parachute" fashion. This anastomosis is with 7-0 Prolene suture.
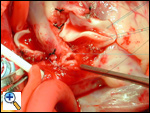
The anastomosis is nearly complete anteriorly. Once the anastomosis is completed, the aortic cross clamp is removed and both right and left coronary arteries are directly perfused by the aorta.
Pulmonary Artery Reanastomosis
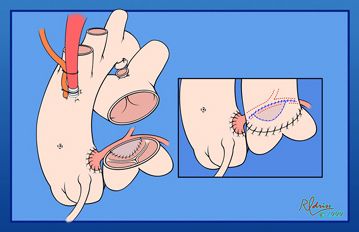
The aortic cross clamp is now off. The posterior sinus of the pulmonary artery where the button was harvested is reconstructed with a patch of fresh autologous pericardium. The pulmonary artery is reanastomosed at the site of the transection as illustrated in the small inset. Reconstruction of the pulmonary artery with the cross clamp off helps to minimize the aortic cross clamp time. In almost all instances it is possible to perform the entire procedure with two doses of cardioplegia given in the sequence described.
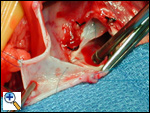 The pericardial patch augmentation is begun at the mid-portion of the base of the pulmonary valve cusp. This reconstruction is with 6-0 Prolene suture. Note the three black ties along the base of the pulmonary valve sinus. These were all significant collaterals that were ligated and divided. This nest of collaterals formed around the orifice of the anomalous left coronary artery and is a relatively common finding.
The pericardial patch augmentation is begun at the mid-portion of the base of the pulmonary valve cusp. This reconstruction is with 6-0 Prolene suture. Note the three black ties along the base of the pulmonary valve sinus. These were all significant collaterals that were ligated and divided. This nest of collaterals formed around the orifice of the anomalous left coronary artery and is a relatively common finding.
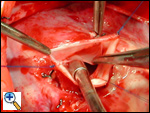 Completed pericardial patch augmentation of the pulmonary valve sinus. Note that the pulmonary valve cusp has been carefully spared and is still completely mobile and effective.
Completed pericardial patch augmentation of the pulmonary valve sinus. Note that the pulmonary valve cusp has been carefully spared and is still completely mobile and effective.
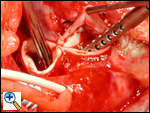 Beginning the pulmonary artery reanastomosis. The pulmonary artery was completely transected, the right and left pulmonary arteries mobilized, and the ligamentum ligated and divided. The anastomosis is with running 6-0 Prolene suture. A portion of the anastomosis will incorporate the pericardial patch used to augment the sinus where the anomalous left coronary artery was harvested.
Beginning the pulmonary artery reanastomosis. The pulmonary artery was completely transected, the right and left pulmonary arteries mobilized, and the ligamentum ligated and divided. The anastomosis is with running 6-0 Prolene suture. A portion of the anastomosis will incorporate the pericardial patch used to augment the sinus where the anomalous left coronary artery was harvested.
Weaning from Cardiopulmonary Bypass
As the patient is warmed, inotropic support is begun. All patients are started on dopamine and dobutamine, initially at 5 µg/kg/minute. Milrinone is also administered at 0.5 mg/kg/minute. The left atrial pressure is monitored with a Camino catheter that is placed through the vent site in the right superior pulmonary vein. The patient is weaned from cardiopulmonary bypass by occluding slowly the venous return and allowing the left side of the heart to fill. It is important to realize that the function by echocardiogram always looks very poor and unimproved from preoperative echocardiographic study. The critical numbers to watch are the systolic blood pressure and the left atrial pressure. If the left atrial pressure is consistently above 20 mm Hg, the patient is unlikely to be able to be weaned from cardiopulmonary bypass and thought should be given toward initiating ECMO or LVAD therapy. Once the patient is successfully weaned from cardiopulmonary bypass, modified ultrafiltration is performed for 10 minutes. Modified ultrafiltration has been shown in several series to increase the myocardial contractility, decrease the central venous pressure, and reduce myocardial edema. Consideration can be given at this point to sealing the mediastinum with a silastic skin patch, rather than approximating the sternum, if there is significant myocardial swelling and/or lung edema. Once the patient is transferred to the intensive care unit, we keep them sedated, paralyzed, intubated and ventilated for at least 3 to 5 days. It is during this time period that they remain relatively unstable because of a very marginal cardiac output. This appears to transition them to a point where they can be successfully taken off pancuronium and then very slowly weaned from ventilatory support. Most of our patients are intubated and ventilated for 10 to 14 days, and our mean hospital stay is 29 days.
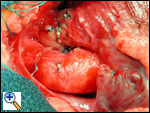 The completed aortic implantation and the completed pulmonary artery reconstruction. All of the cannulation sites have been tied, the patient is off cardiopulmonary bypass, and the chest is ready to be closed.
The completed aortic implantation and the completed pulmonary artery reconstruction. All of the cannulation sites have been tied, the patient is off cardiopulmonary bypass, and the chest is ready to be closed.
Preference Card
Equipment/Instruments
- Transesophageal echocardiography
- Potential for ECMO standby
- Single-use Bear vessel clamps (AROSurgical Instruments Corporation, Newport Beach, CA )
- Camino monitor and pressure monitoring kit (Integra NeuroSciences, Plainsboro, NJ)
Sutures
- 6-0 and 7-0 Prolene monofilament suture (Ethicon, Somerville, NJ)
Tips & Pitfalls
- The surgical strategy of bicaval venous cannulation, left ventricular vent, moderate hypothermia (28ºC), snares on the right and left pulmonary arteries, and two doses of antegrade cardioplegia is an attempt to provide maximal myocardial preservation during the procedure.
- For postoperative inotropic support we routinely use dopamine, dobutamine, and milrinone. Dopamine is started at 5-10 µg/kg, dobutamine at 5-10 µg/kg, and milrinone at 0.5 µg/kg/min. All patients in our series are kept paralyzed, intubated, and ventilated for at least 3-5 days following the procedure while they have slow improvement of their myocardial function.
- Many of these patients have significant collateral formation around the anomalous coronary artery. Careful ligation and division of these small collateral vessels prior to or shortly after starting cardiopulmonary bypass will minimize the risk of requirement of reinstitution of cardiopulmonary bypass to control bleeding following the procedure. One patient in our series required reinstitution of cardiopulmonary bypass twice because of significant bleeding from these collaterals posterior to the reconstructed pulmonary artery.
- Left atrial pressure is monitored with a Camino catheter placed through the right superior pulmonary vein vent site. Watching the left atrial pressure and its response to volume loading and inotrope manipulation is helpful in weaning from cardiopulmonary bypass. We typically use the left atrial Camino catheter for the first 30 minutes following weaning from cardiopulmonary bypass. Some centers recommend monitoring the left atrial pressure for many days following the procedure, although we have not used that strategy.
- These patients often have mild-to-moderate or even moderate-to-severe mitral valve insufficiency. In our series we have not performed mitral valve repair at the time of the anomalous coronary artery implantation in any patient. In almost all patients the mitral insufficiency will improve over a period of time. Unless the patient is unable to be weaned from cardiopulmonary bypass, we would not consider mitral valve repair or replacement at the time of aortic implantation.
- If the patient is not able to be weaned from cardiopulmonary bypass, little time should be spent converting the patient to either ECMO or left ventricular assist device support. ECMO can be instituted by either using the ascending aorta and right atrial appendage through a silastic skin patch or by cannulating the neck vessels, which were prepped at the time of the original prepping and draping. A ventricular assist device can also be placed through the sternotomy incision. We have not yet required ECMO in our series.
- Some patients with significant myocardial swelling may benefit from use of a temporary silastic skin patch for 48-72 hours following the procedure. This allows time for resolution of myocardial edema or pulmonary edema, and chest closure may be performed in the intensive care unit several days postoperatively. We have used delayed sternal closure in one patient in our series.
Results
From 1989 through 2003, 24 consecutive children (median age, 0.45 years) underwent aortic implantation for ALCAPA at Children’s Memorial Hospital . A similar surgical strategy was used for all patients as outlined in the Techniques Section. All patients survived. No patient was placed on ECMO or LVAD. Mean cardiopulmonary bypass time was 150 minutes; mean aortic cross clamp time was 45 minutes. Mean time from cross clamp removal to cardiopulmonary bypass off (weaning time) was 60 minutes. One patient in the series had delayed sternal closure. Mean hospital stay was 29 days. Excellent results with aortic implantation have been reported by other surgeons. Results of these series are illustrated in Table 1. The overall mortality of aortic implantation for ALCAPA is less than 10 percent.
Table 1. Results of Aortic Implantation for ALCAPA
| Author | Year | No. of Patients |
Mortality (%) |
| Neirotti | 1991 | 12 | 0 |
| Vouhé | 1992 | 31 | 5 (16) |
| Alexi-Meskishvili | 1994 | 11 | 0 |
| Turley | 1995 | 11 | 0 |
| Lambert | 1996 | 34 | 5 (15) |
| Backer | 2003 | 24 | 0 |
| Azakie | 2003 | 47 | 4 (8) |
| TOTAL | 170 | 14 (8.2) | |
References
- Backer CL, Hillman N, Dodge-Khatami A, et al: Anomalous origin of the left coronary artery from the pulmonary artery: successful surgical strategy without assist devices. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2000;3:165-72.
- Backer CL, Stout MJ, Zales VR, et al. Anomalous origin of the left coronary artery. A twenty-year review of surgical management. J Thorac Cardiovasc Surg 1992;103:1049-58.
- Vouhé PR, Tamisier D, Sidi D, et al. Anomalous left coronary artery from the pulmonary artery: results of isolated aortic reimplantation. Ann Thorac Surg 1992;54:621-7.
- Alexi-Meskishvili V, Hetzer R, Weng Y, et al. Anomalous origin of the left coronary artery from the pulmonary artery. Early results with direct aortic reimplantation. J Thorac Cardiovasc Surg 1994;108:354-62.
- Turley K, Szarnicki RJ, Flachsbart KD, et al. Aortic implantation is possible in all cases of anomalous origin of the left coronary artery from the pulmonary artery. Ann Thorac Surg 1995;60:84-9.
- Lambert V, Touchot A, Losay J, et al. Midterm results after surgical repair of the anomalous origin of the coronary artery. Circulation 1996;94:II-38-II-43.
- Del Nido PJ, Duncan BW, Mayer JE Jr, et al. Left ventricular assist device improves survival in children with left ventricular dysfunction after repair of anomalous origin of the left coronary artery from the pulmonary artery. Ann Thorac Surg 1999;67:169-72.
- Neirotti R, Niyveld A, Ithuralde M, et al. Anomalous origin of the left coronary artery from the pulmonary artery: repair by aortic reimplantation. Eur J Cardiothorac Surg 1991;5:368-72.
- Dodge-Khamati A, Mavroudis C, Backer CL. Anomalous origin of the left coronary artery from the pulmonary artery: collective review of surgical therapy. Ann Thorac Surg 2002;74:946-55.
- Azakie A, Russell JL, McCrindle BW, et al. Anatomic repair of anomalous left coronary artery from the pulmonary artery by aortic reimplantation: early survival, patterns of ventricular recovery and late outcome. Ann Thorac Surg 2003;75:1535-41.