ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Branched Stentgrafts for the Treatment of Thoracoabdominal Aortic Aneurysms
Patient Selection
Patients at high risk for open repair of thoracoabdominal aortic aneurysms (TAAA) are often relegated to medical therapy. An alternative therapy involves extra-anatomic bypass followed by endovascular stentgrafting of the entire thoracic and abdominal aorta with standard thoracic stentgrafts [1]. This hybrid approach still carries a high risk because the “debranching” portion of the operation is extensive. The development of newer branched devices have allowed for a purely endovascular repair and will be the subject of this manuscript [2].
Indications for endovascular repair of TAAA include:
- Presence of an aortic aneurysm at higher risk for rupture than the risk of repairing it using a branched stentgraft (greater than 6 cm).
- Relatively high risk for open surgical repair based on a thorough assessment of comorbidities and an understanding that the durability of these endovascular devices is yet unknown.
- Anatomy conducive to stentgrafting including a) a normal caliber proximal segment aortic segment, and b) a distal landing zone.
Patients whose comorbidities predict a life-expectancy not warranting aneurysmal repair and who otherwise do not meet the indications above are contraindicated to undergo endovascular repair. Furthermore, patients with iliac lesions not amenable to angioplasty or recanalizable to allow passage of the delivery system are contraindicated. Other contraindications include allergies to device materials such as stainless steel or polyester graft material, and systemic infection. All patients should be able to undergo the required follow up imaging. Chronic aortic dissection is a relative contraindication with the current devices and techniques.
Operative Steps
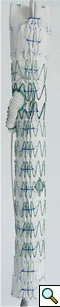 |
| Figure 1 |
Device (Not FDA approved)
The branched TAAA stentgrafts are a modular system based on the Zenith stentgraft (Cook Inc, Bloomington, Indiana). The main body component is custom designed straight or tapered device with reinforced fenestrations or directional helical branches precisely constructed to correlate with the patients target vessels (Figure 1). Reinforced fenestrations are mated to balloon expandable covered stents (Jomed, Abbott Labs, Abbott Park, Illinois) and the directional branches are mated to self-expanding covered stents (Fluency, Bard, Inc, Tempe, Arizona).
 |
| Figure 2 |
Planning
A clear understanding of the aortic, iliac and femoral anatomy based on preoperative imaging is critical to proper patient selection, device design, and accurate delivery. Other important anatomic details include a clear understanding of the relationships of all of the visceral and renal branch arteries to one another and their specific clockwise orientation to the aortic axis, the proximal seal zone, and aortic bifurcation (Figure 2). A site of delivery of the main body must be selected based on the size, extent of calcification and tortuosity of the access vessels. The procedures cannot be performed without accurate preoperative planning with the use of high resolution imaging and three-dimensional reconstruction techniques to be performed by the operator.
Operating Room
An endovascular suite with a fixed imaging system with 12 to 16 inch image intensifier is best for the multiple views and occasionally prolonged imaging necessary for this complex procedure. Iliac access is necessary in up to 10% of patients and so the suite should be amenable to the performance of an open operation. We have performed over 50% of these procedures using regional anesthesia due to the severity of pulmonary disease in many of these patients but general anesthesia is preferred because of the length of time and need for patient cooperation with imaging. Patients requiring extensive coverage of the aorta or otherwise at high risk for spinal cord injury should have cerebrospinal fluid drainage.
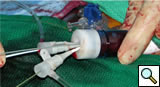 |
| Figure 3 |
Deployment
The operator needs to re-familiarize himself with the specific device design especially regarding the location of the fenestrations in relation to the edge of the graft material and to one another and also the predetermined access.
Bilateral common femoral arteries are exposed and access obtained on the side of delivery. The catheter is then directed to the ascending aorta and exchanged for a stiff guidewire (0.035 Amplatz or Lunderquist). A large bore (20-24F Check Flow) sheath is placed in the contralateral femoral vessel and multiply punctured with short 5F sheaths (Figure 3).
Once bilateral wire access has been obtained, the patient is heparinised. A straight angiography catheter is then positioned at the level of the diaphragm and angiography performed to delineate the visceral segment.
The device is then oriented ex vivo. The device is then advanced through the femoral artery to the level of the visceral segment and aligned with the appropriate branch arteries. Doing so may require multiple low-dose contrast injections.
It is important to also verify that the distal aspect of the device is above the aortic bifurcation. Once orientation of the proximal body has been confirmed, the top two stents are partially deployed. The device position is then adjusted as necessary in both the longitudinal and rotational planes. The sheath is then completely withdrawn to expand the stentgraft throughout its length. At this point the device is only partially expanded due to the presence of constraining wires. The next step entails cannulation of the fenestrations. The contralaterally placed wire and angiocath should be withdrawn at this point and the main body of the device recannulated. Using an appropriately angled catheter and flexible angled guide wire, at least two fenestrations and their corresponding arteries should be cannulated. With catheter access to the target artery the wire should be exchanged for a stiffer access wire (Rosen). An access sheath (7Fr Ansel) or guiding catheter (8Fr MPB) should then be advanced through the fenestration into the artery. This step should be repeated for at least two of the branch arteries before releasing the constraining wire.
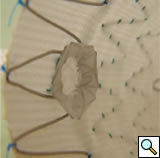 |
| Figure 4 |
Stenting the branch vessels can now be performed via the previously placed access sheaths. Balloon expandable covered stents measuring 18-38mm in length and 6-9mm in diameter are deployed on 7mm x 4cm or 8mm x 4cm balloons mostly into the target vessel with approximately 3mm extending proximally into the aorta. The aortic portion of the stentgrafts should then be flared with a 10mm x 2cm balloon to rivet them in place within the main device (Figure 4). Further flaring with a larger compliant balloon (Coda) is selectively employed. Each stent should be completely deployed including the flaring process before proceeding to the subsequent one.
Some devices are designed with a combination of fenestrations and helical directional branches. These latter configurations are preloaded with wires to allow for easier access and are conjoined to the target branch vessels with self-expanding stentgrafts because their construct allows for a longer overlap and seal. Newer iterations of these devices have multiply preloaded branches and a unilateral delivery system.
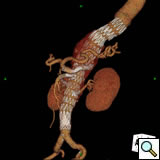 |
| Figure 5 |
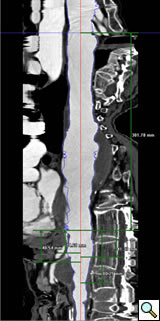
Follow-Up
The perioperative complications of TAAA repair have not been eliminated but their incidence is reduced and include renal dysfunction, respiratory failure, stroke, MI, and spinal cord injury [2]. Other late complications include endoleaks and therefore all patients need regularly scheduled follow-up CT imaging at months 1, 6, 12 and annually (Figure 5).
Preference Card
Sheaths
- Multiple short 5Fr.
- 20 – 24Fr Check Flow.
- 7Fr Ansel.
- 8Fr Guides, RDC and other curves.
Catheters
- Straight Omniflush.
- 5Fr Angled Glide.
- 5Fr in multiple shapes including KMP, C2, VS1.
Guidewires
- 0.035 Starter.
- 0.035 Floppy Glide.
- 0.035 Rosen.
- 0.035 Lunderquist.
- Micro system – 0.018 Glide Gold, 0.014 Balanced Middle Weight, 0.014 Iron Man in concert with Renegade micro catheter.
Balloons
- 7mm x 4cm or 8mm x 4cm Optipro or equivalent for branch stentgraft delivery through fenestrations.
- 10mm x 2cm or 12mm x 2cm Optipro or equivalent for proximal flaring of branches.
- 40mm Coda compliant balloon.
Stentgrafts
- Aortic custom-designed component.
- Zenith TX2 thoracic or infrarenal standard commercial components for proximal or distal extension.
- Jomed 4-9mm or 6-12mm balloon expandable stentgrafts in variable lengths for fenestrated branches.
- Fluency 8, 10, or 12mm self expanding stentgrafts in variable lengths for directional branches.
Miscellaneous
- Cook hydrophilic dilator set (20-24Fr).
- Torque devices.
- Access needles.
- Closure devices.
- Occasional giant Palmaz stent.
Tips & Pitfalls
- Be certain to select patients healthy enough to tolerate the procedure as it is still a thoracoabdominal aneurysm repair with potential for serious complications.
- Cannot be done without 3D planning and repeat planning and confirmation of the plan. In the OR, trust the plan.
- Often helpful to obtain access to the branch vessels prior to main body device deliver to assist with positioning.
- Get complete access into at least two branches through the partially deployed device prior to full deployment.
- It is OK to stop and come back another day if not all of the branches are engaged.
References
- Resch T, Greenberg RK, Lyden S, et al. Combined staged procedures for the treatment of thoracoabdominal aneurysms. J Endovasc Ther 2006;13:481-89.
- Roselli EE, Greenberg RK, Pfaff K, Francis C, Svensson LG, Lytle BW. Endovascular treatment of thoracoabdominal aneurysms. J Thorac Cardiovasc Surgery 2007;133:1474-82.




