ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Laparoscopic Diaphragmatic Plication
Podgaetz E, Andrade R, Jr. RGC. Laparoscopic Diaphragmatic Plication. October 2022. doi:10.25373/ctsnet.21397356
Laparoscopic diaphragmatic plication is a safe and effective operation in carefully selected patients. Laparoscopic diaphragm plication was first reported by Hüttl et al in three patients (1). The authors’ previously published experience demonstrated significant improvement in dyspnea, quality of life, and pulmonary function tests in patients with unilateral diaphragm paralysis or eventration (2).
The approach for hemidiaphragm plication should be individualized by patient anatomy, comorbidities, and surgeon experience. Regardless of approach, proper patient selection, safety, and a tight imbrication of the entire hemidiaphragm are essential.
Indications
Prospective candidates for diaphragmatic plication must have dyspnea that cannot be solely attributed to another process (i.e., poorly controlled primary lung or heart disease), and must have an elevated hemidiaphragm on a PA/LAT chest x-ray. Since the only goal of diaphragm plication is to treat dyspnea, operative intervention is indicated exclusively for symptomatic patients. An elevated hemidiaphragm or paradoxical motion per se does not merit surgery in the absence of significant dyspnea.
Contraindications and Complications
Relative contraindications to a laparoscopic approach to diaphragm plication include: previous extensive abdominal surgery, BMI greater than 35, and comorbidities that may worsen with pneumoperitoneum (e.g., chronic renal failure, history of deep venous thrombosis). Morbidly obese patients pose particular technical challenges due to hepatomegaly from steatosis, or excessive omental fat in the left upper quadrant.
Preoperative Planning
The diagnosis of symptomatic hemidiaphragm paralysis or eventration is primarily clinical, and relies mostly on history, chest x-ray, and the physician’s clinical acuity. Preoperative pulmonary function tests (PFTs) provide relative objectivity to the assessment of dyspneic patients with an elevated hemidiaphragm. However, PFTs are imprecise and do not correlate well with severity of dyspnea, or response to plication. Diaphragm dysfunction reduces the compliance of the chest wall. Hence, a restrictive pattern (i.e., low forced vital capacity [FVC] and low forced expiratory volume in one second [FEV1]) is the norm.
Imaging
CXR:
On a standard full-inspiratory postero-anterior and lateral (PA/LAT) chest x-ray, the right hemidiaphragm is normally 1-2 cm higher than the left.
Sniff Test:
During fluoroscopy, patients are instructed to sniff, and diaphragmatic excursion is evaluated. Normally, the diaphragm moves caudally, but in patients with hemidiaphragmatic paralysis, the diaphragm may (paradoxically) move cranially. Patients with diaphragmatic eventration, however, may also exhibit passive upward movement of the diaphragm when sniffing.
CT Scan:
The main utility of CT scans is to rule out the presence of a cervical or intrathoracic tumor as the cause of phrenic nerve paralysis. CT scans may also be used to evaluate the possibility of an infra- or supradiaphragmatic process as the cause of hemidiaphragm elevation.
Surgical Technique
Anesthesia:
The procedure is performed under general anesthesia, with a single-lumen endotracheal tube. Selective ventilation is not necessary.
Position:
The patient is placed in the supine position with arms abducted. The abdomen and lower lateral chest wall are prepared and draped to allow access for chest tube placement. A foot board is essential for steep Trendelenburg positioning.
Operative Technique:
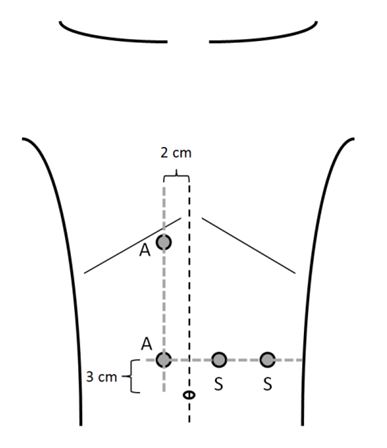
- Ports: The authors use four 12 mm ports. Two assistant ports are placed 2 cm parallel to the midline on the opposite site of the elevated hemidiaphragm. The two working ports are placed in the ipsilateral upper quadrant (Figure 1). The abdomen is insufflated with CO2 to a pressure of 15 mmHg.
- Exposure: Steep reverse Trendelenburg positioning helps optimize exposure of the posterior portion of the hemidiaphragm. For a right-sided plication, transection of the falciform ligament is useful for appropriate access to the diaphragm. The thinned-out hemidiaphragm is taut and displaced cranially as a result of pneumoperitoneum (Figure 2A). A small perforation is made at the dome of the diaphragm with electrocautery (Figure 2B). The resulting pneumothorax releases the tension on the hemidiaphragm, and allows the surgeon to easily pull the hemidiaphragm into the abdominal cavity for suturing (Figures 2C, 2D). At this point, the authors often place a 19 Blake pleural drain through an incision in the anterolateral chest wall to vent the pneumothorax as needed.
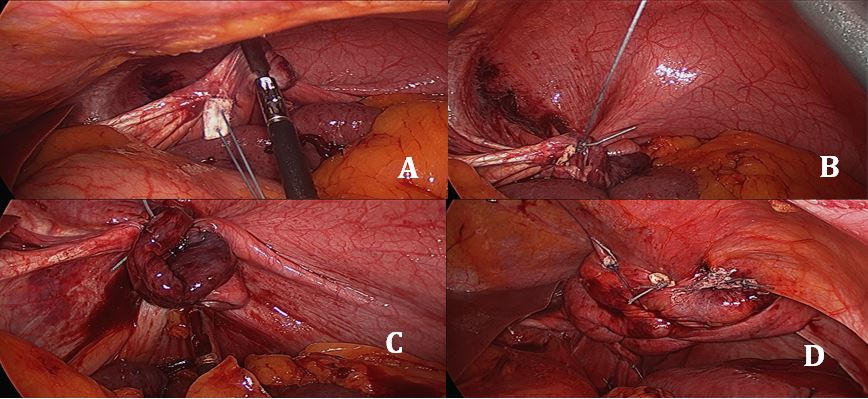
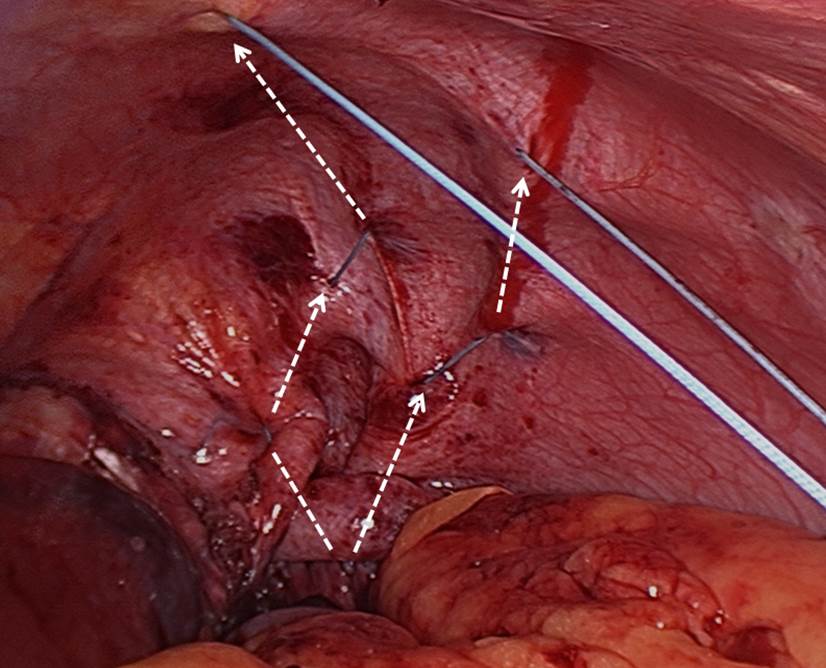
- Stitching: The authors use pledgeted U-stitches (#2 non-absorbable, braided suture, 31 mm curved needle). The first stitch is placed centrally and as far posteriorly as possible (Figure 3A). Traction on the first stitch facilitates exposure for two or three subsequent deeper stitches to plicate the posterior portion of the hemidiaphragm in an anteroposterior direction (Figures 3B, 3C, 3D). To plicate the anterior portion of the hemidiaphragm, two to three weaving stitches are placed (Figure 4). The diaphragm must be taut at the end of the procedure. Closure of the initial perforation at the dome occurs with the plication.
- Tube Thoracostomy: The authors leave the pleural drain in place upon completion of the procedure, and verify that it has not been caught in a stitch.
- Intraoperative Management of Lower Lobe Atelectasis: Upon completion of the plication, the authors ask the anesthesia team to ventilate the patient with high tidal volumes and a PEEP of 10 cm H2O until extubation, with the intention of re-expanding the lower lobe. If respiratory secretions are copious after recruitment, flexible bronchoscopy should be performed.
Figure 1: Port placement for left-sided hemidiaphragm
plication.
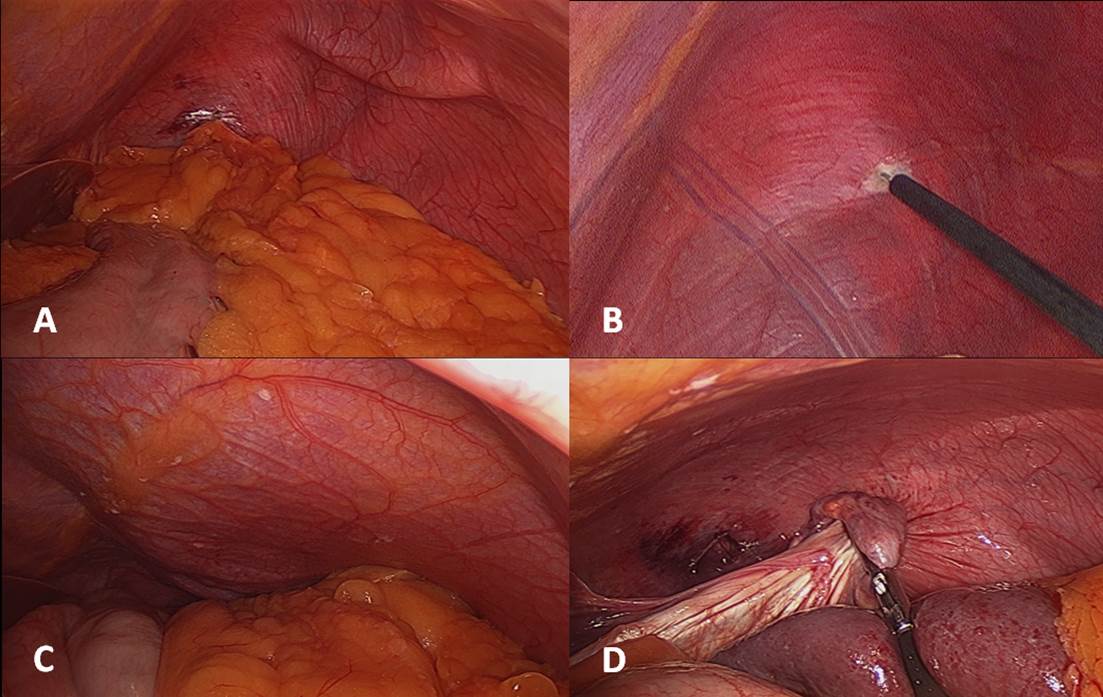
Figure 2: Technique for diaphragmatic exposure.
Figure 3: Technique for suture placement and antero-
posterior plication.
Post-procedure Care
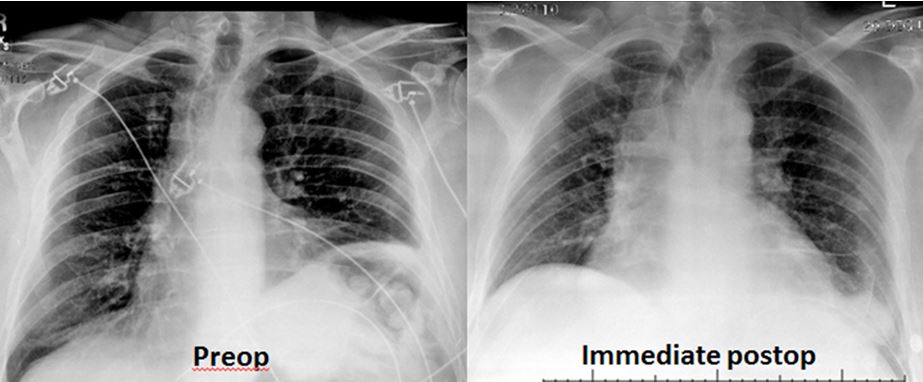
Postoperatively, patients participate in intense pulmonary toilet to re-expand the lower lobe of the ipsilateral lung. The chest drain remains in place until output is less than 200 mL/day. Occasionally patients need to be discharged with the chest tube in place. Premature removal of the chest drain can lead to symptomatic pleural effusion with recurrent lower-lobe atelectasis. The immediate postoperative chest x-ray should show that the plicated side is lower than the opposite side, with an acute costophrenic angle, and that the opposite side is actually elevated in comparison to the preoperative chest x-ray (Figure 5). Patients are monitored with the St. George’s Respiratory Questionnaire (SGRQ), PA/LAT chest x-ray, and PFTs at one month after discharge and yearly thereafter.
Complications
Complications of laparoscopic plication include: prolonged chest tube drainage of greater than seven days (8%), respiratory failure (4%), gastrointestinal hemorrhage (4%), splenic laceration requiring splenectomy (4%), stroke (4%), and atrial fibrillation (4%) (3). The severity of some of these complications is probably also a reflection of the severity of comorbidities in this patient population.
References
- Hüttl TP, Wichmann MW, Reichart B, Geiger TK, Schildberg FW, Meyer G. Laparoscopic diaphragmatic plication: long-term results of a novel surgical technique for postoperative phrenic nerve palsy. Surgical endoscopy. 2004;18:547-51.
- Groth SS, Rueth NM, Kast T, D'Cunha J, Kelly RF, Maddaus MA, et al. Laparoscopic diaphragmatic plication for diaphragmatic paralysis and eventration: an objective evaluation of short-term and midterm results. The Journal of thoracic and cardiovascular surgery. 2010;139:1452-6.
- Groth SS, Andrade RS. Diaphragm plication for eventration or paralysis: a review of the literature. The Annals of thoracic surgery. 2010;89:S2146-50.
Disclaimer
The information and views presented on CTSNet.org represent the views of the authors and contributors of the material and not of CTSNet. Please review our full disclaimer page here.





Comments