ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Nontraumatic Chylothorax in the Setting of Superior Vena Cava Stenosis
Bonanno A, Schenning R, Tieu B. Nontraumatic Chylothorax in the Setting of Superior Vena Cava Stenosis. February 2019. doi:10.25373/ctsnet.7674278.
Introduction
Chylothorax is a rare but potentially morbid complication following thoracic surgery. Nontraumatic chylothorax is even less common and typically is due to malignancy, congenital or idiopathic disorders of the lymphatic system, systemic diseases, and infection [1]. Here, the authors present a case of nontraumatic chylothorax due to central venous stenosis.
Case Presentation
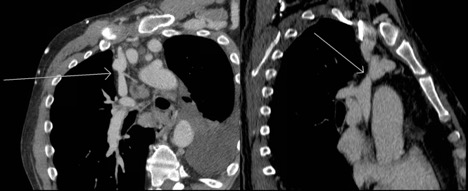
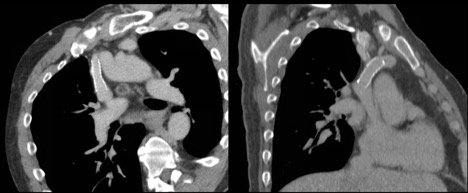
Figure 1. Oblique reformatted chest CT demonstrating nonocclusive stenosis of the distal portion of the SVC. Note the synechiae or "web" within the innominate vein.
A 57-year-old man with a history of renal failure and previous renal transplantation complicated by rejection requiring dialysis presented to the emergency room with the acute onset of dyspnea, nonproductive cough, and an inability to lay flat. He had a history of prior left tunneled internal jugular dialysis catheter placement and left upper extremity dialysis fistula, which had previously required multiple fistulagrams for prolonged bleeding and elevated venous pressures. During examination, his chest x-ray showed a large left pleural effusion and he underwent thoracentesis by the emergency medicine team with evacuation of 2 L of turbid fluid.
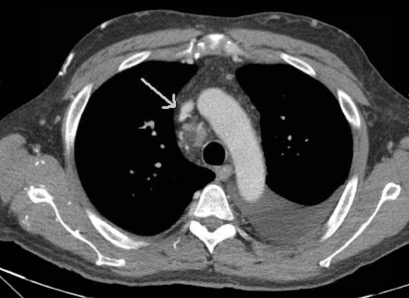
Figure 2. Axial chest CT demonstrating occlusive stenosis of the central portion of the innominate vein and SVC confluence.
Given his chronic immunosuppression, he was admitted by internal medicine for initiation of intravenous (IV) antibiotics. Thoracic surgery, transplant infectious disease, and nephrology were consulted following admission. He underwent an echocardiogram, which did not demonstrate congestive heart failure. A computed tomography (CT) scan demonstrated right internal jugular occlusion, superior vena cava (SVC) stenosis, prominent thoracic venous collaterals, and cervical and mediastinal lymphadenopathy (Figures 1 and 2). His pleural fluid cytology and cultures were negative, but testing indicated the presence of chylomicrons and his triglyceride level was 1154 mg/dL, which was consistent with a chylothorax.
His IV antibiotics were discontinued, and he was placed on a medium chain triglyceride diet with octreotide three times daily. A cervical lymph node biopsy was negative for lymphoma, and he was monitored closely as an outpatient for recurrence. He continued to reaccumulate chylous fluid despite dietary modifications and required additional thoracenteses.
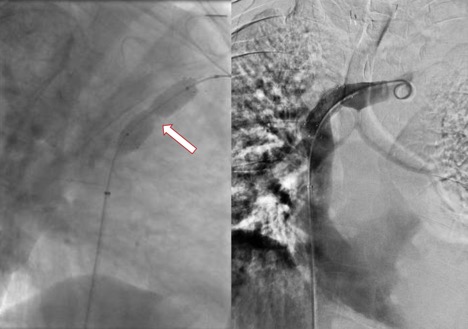
Figure 3. Stent deployment using a 14 x 40 mm self-expanding nitinol stent across the stenotic portion of the peripheral SVC before (left) and after (right) balloon dilation of the stent. Note the robust waist of the angioplasty balloon corresponding to the critical stenosis on preprocedural corss-sectional imaging. The initial pressure gradient across the stenosis was 21 mm Hg. Following stent deployment the pressure gradient was 1 mm Hg.
Subsequently, the decision was made to proceed with a venogram and possible SVC stent placement. If this were unsuccessful, he would undergo thoracoscopic thoracic duct ligation. He was found to have hemodynamically significant stenosis of the superior SVC resulting in a 21 mm Hg pressure gradient between the left innominate vein and central SVC. He underwent successful stent placement with decompression of previously visualized left thoracic venous collaterals (Figure 3). After stent placement the pressure gradient was reduced to 1 mm Hg. The patient subsequently has remained stable, with resolution of his chylothorax without the need for further interventions.
Discussion
Nontraumatic chylothorax is a rare condition with etiologies including malignancy, congenital or idiopathic disorders of the lymphatic system, systemic diseases, and infection [1]. The diagnosis is made by findings of milky-appearing pleural fluid and triglyceride counts more than 110 mg/dL with the presence of chylomicrons [2]. Initial treatment is conservative management with diet modification and pleural drainage [1]. Pleurodesis, surgical ligation of the thoracic duct, and thoracic duct embolization are reserved for those patients for whom conservative management is not successful. However, given the high variability in etiologies of nontraumatic leaks, treatment may be more challenging [1].
Stricture of the SVC, in particular, is a rare acquired abnormality that can result from both intrinsic and extrinsic factors [3]. Intrinsic causes include prolonged indwelling central venous catheters, pacemakers, and postoperative effects. Intimal irritation and inflammation leads to intimal hyperplasia followed by severe narrowing or occlusion of the SVC [4]. This more commonly will produce symptoms of SVC syndrome, which is characterized by edema of the upper extremities, head, and neck. Subsequently, there is extensive development of venous collaterals that bypass the obstructed segment [3].Patients on hemodialysis via an arteriovenous (AV) fistula, even without SVC stenosis, may also develop venous congestion and multiple collaterals. The creation of an AV fistula produces arterial flow directly into the venous circulation, which in turn results in a decrease in the systemic vascular resistance in blood pressure [5]. This then generates an increase in circulating angiotensin II, aldosterone, and arginine vasopressin, which increase heart rate, cardiac contractility, total blood volume, ventricular preload, stroke volume, and cardiac output. These elevated pressures and total blood volume can eventually lead to systemic venous congestion, and with a patient who has SVC stenosis, a heightened amount of congestion with the formation of collaterals [5]. With increasing pressure, there is a resultant obstruction in forward lymphatic flow and high pressures within small lymphatic channels, causing spontaneous rupture and subsequent chyle leak [1]. This is the suspected etiology of chylothorax in our patient, because he developed elevated venous pressures leading to formation of multiple collaterals and subsequent chyle leak due to increased pressure within the lymphatic system.
There have been few case reports demonstrating the association of chylothorax and SVC stenosis. One such case described central venous obstruction due to thrombus causing recurrent chylothorax in a 6-month-old girl following surgical repair of tetralogy of Fallot [6]. Despite maximal medical therapy and surgical ligation of the thoracic duct, her chylothorax persisted. Her chylothorax finally resolved after stent implantation of the SVC and innominate vein. In another example, similar to the authors’ patient, a 34-year-old patient on hemodialysis who presented with facial swelling and dyspnea was found to have chylothorax and chylopericardial tamponade due to stenosis of the SVC and right brachiocephalic vein in the setting of an indwelling tunneled catheter [7]. This patient successfully underwent angioplasty of the SVC stenosis with subsequent resolution of his effusions.
As was mentioned above, treatment of chylothorax initially includes pleural drainage and dietary modifications that can include a low fat (medium chain triglyceride) or nonfat diet, or nil per os and total parenteral nutrition [8]. Some patients may also benefit from administration of octreotide [9]. However, if conservative management fails, early surgical intervention to include ligation of the thoracic duct or lymphoscintigraphy with thoracic duct embolization is recommended given the increased morbidity associated with prolonged chylous effusion [8]. In cases of central venous obstruction such as SVC stenosis, these treatments may fail as the primary issue is more proximal. Therefore, initial endovascular intervention can include angioplasty and/or SVC stent placement in order to decrease chylous output. Angioplasty alone has been thought to lead to recurrence of stenosis due to endothelial injury; however, long-term overall patency rates are similar for both primary stenting and angioplasty [10]. If endovascular approaches fail, possibilities for patients with AV fistulas as a primary cause of exacerbation can include ligation of the fistula. Surgical intervention can also include SVC reconstruction for nonmalignant disease by using saphenous vein graft, human allograft, and expanded polytetrafluoroethylene if thoracic duct ligation is not successful [11].
In conclusion, nontraumatic chylothorax is rare and can be associated with SVC stenosis. Resultant arterial pressurization of the left upper extremity and central venous system in conjunction with the central venous stenosis in the authors’ patient was likely the cause of his chylothorax. Traditional initial treatment with conservative management and/or thoracic duct ligation may not be successful with this underlying pathophysiology. Consideration of angioplasty or stenting is a reasonable initial management following conservative measures including pleural drainage and diet modifications.
References
- Nadolski G. Nontraumatic chylothorax: diagnostic algorithm and treatment options. Tech Vasc Interv Radiol. 2016;19(4):286-290.
- LaPar DJ, Mery CM, Turek JW, eds. TSRA Review of Cardiothoracic Surgery. 2nd ed. Scotts Valley, CA: CreateSpace; 2016.
- Sonavane SK, Milner DM, Singh SP, Abdel Aal AK, Shahir KS, Chaturvedi A. Comprehensive imaging review of the superior vena cava. Radiographics. 2015;35(7):1873-1892.
- Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146(2):259-261.
- Narechania S, Tonelli AR. Hemodynamic consequences of a surgical arteriovenous fistula. Ann Am Thorac Soc. 2016;13(2):288-291.
- Tamai A, Kurishima C, Seki M, Masutani S, Taketazu M, Senzaki H. Stent implantation for effective treatment of refractory chylothorax due to superior vena cava obstruction as a complication of congenital cardiac surgery. Clin Med Insights Cardiol. 2012;6:97-101.
- Adekile A, Adegoroye A, Tedla F, Levin D, Salifu MO. Chylothorax and chylopericardial tamponade in a hemodialysis patient with catheter-induced superior vena cava stenosis. Semin Dial. 2009;22(5):576-579.
- Martucci N, Tracey M, Rocco G. Postoperative chylothorax. Thorac Surg Clin. 2015;25(4):523-528.
- Ismail NA, Gordon J, Dunning J. The use of octreotide in the treatment of chylothorax following cardiothoracic surgery. Interact Cardiovasc Thorac Surg. 2015;20(6):848-854.
- Bakken AM, Protack CD, Saad WE, Lee DE, Waldman DL, Davies MG. Long-term outcomes of primary angioplasty and primary stenting of central venous stenosis in hemodialysis patients. J Vasc Surg. 2007;45(4):776-783.
- Kalra M, Gioviczki P, Andrews JC, et al. Open surgical and endovascular treatment of superior vena cava syndrome caused by nonmalignant disease. J Vasc Surg. 2003;38(2):215-223.