ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Pulmonary Valve Replacement in Adult Congenital Cardiac Surgery
Introduction
In this video, the authors demonstrate how to replace the pulmonary valve in adult patients with severe pulmonary valve regurgitation (PVR). Most of these adult congenital heart disease patients underwent Tetralogy of Fallot repair in childhood. The transannular patch technique was used to relieve the obstruction at the level of the annulus, which lead to the well-known, long-term problems associated with free pulmonary valve regurgitation (PR), including right ventricular dilation and dysfunction. The right ventricle (RV) changed from a pressure-loaded condition to a volume-overloaded situation, leading to right ventricle dilatation and secondary tricuspid valve insufficiency (TR). Free pulmonary valve regurgitation is the most common cause of progressive RV dysfunction.
Methods
A preoperative transesophageal echocardiography (TOE) is performed during the Valsalva maneuver to determine whether the patient has an intra-cardiac shunt. If a shunt is not present, the procedure can be carried out on the beating heart.
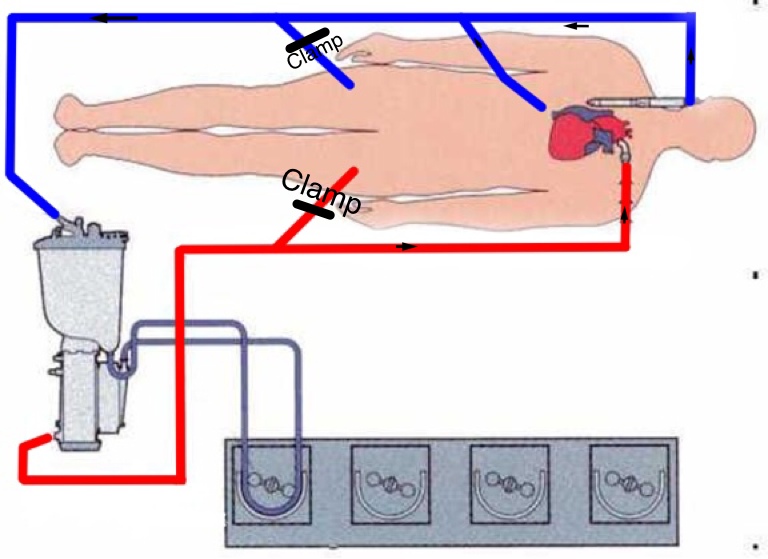
The redo-sternotomy can be technically challenging due to adhesions and the proximity of both the dilated right ventricle and dilated aorta to the sternum. The cardiopulmonary bypass (CPB) circuit is set up to allow femoro-femoral cannulation, as well as standard chest cannulation (Figure 1).
Figure 1: The CPB circuit is set up to allow femoro-femoral cannulation as well as standard chest cannulation. In case of uneventful median sternotomy the CPB is initiated via standard aortic and bicaval cannulation. In this case, the lines for the femoral cannulae are clamped and not used.
16G BD Angiocath™ cannulae are inserted preoperatively in the right femoral vein and in the left femoral artery. The cannulae are used for initiating a percutaneous femoro-femoral CPB if there is significant hemorrhage during the redo-sternotomy.
If the chest opening is uneventful, the ascending aorta and both the caval veins are cannulated and CPB is started. The femoral lines of the CPB circuit are clamped (Figure 1).
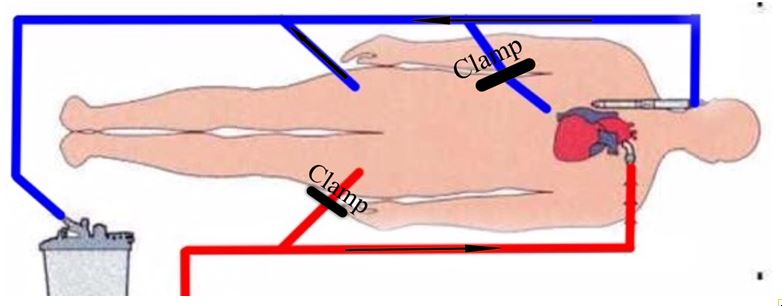
If femoro-femoral CPB is initiated due to complications during the sternotomy the aorta and the superior vena cava (SVC) are cannulated switching the CPB into the chest (Figure 2). The left femoral artery is dissected, decannulated, and repaired to avoid complication due to reduction in distal perfusion. The inferior vena cava (IVC) drainage is supplied by the right femoral venous cannula that is left in place for the rest of the operation (Figure 3).
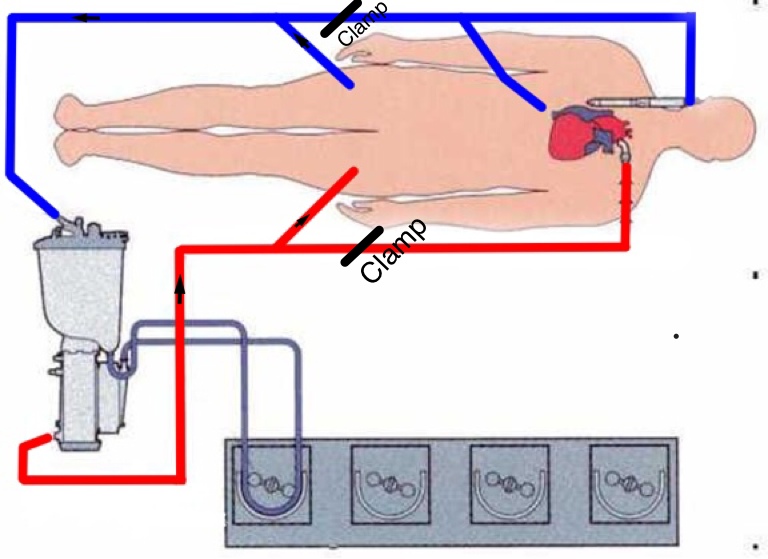
Figure 2: The lines are clamped beyond the femoral lines to allow the extra-thoracic CPB.
If preoperative imaging identifies that the cardiac structure is fused to the sternum, the heart is decompressed by initiating femoro-femoral bypass prior to sternotomy. This is achieved with a cutdown technique. To ensure adequate distal perfusion of the left leg, the femoral artery is cannulated via a conduit (Figure 3).
Figure 3: The CPB, initially started using femoral vessels, is now converted into the chest after the cannulation of the ascending aorta and the SVC. The drainage from the IVC will be guaranteed by the right femoral cannula.
In the authors’ experience, it is very rare to encounter complications during the redo-sternotomy. By using the described technique, the authors have prevented and minimized the need for emergency extra-thoracic CPB.
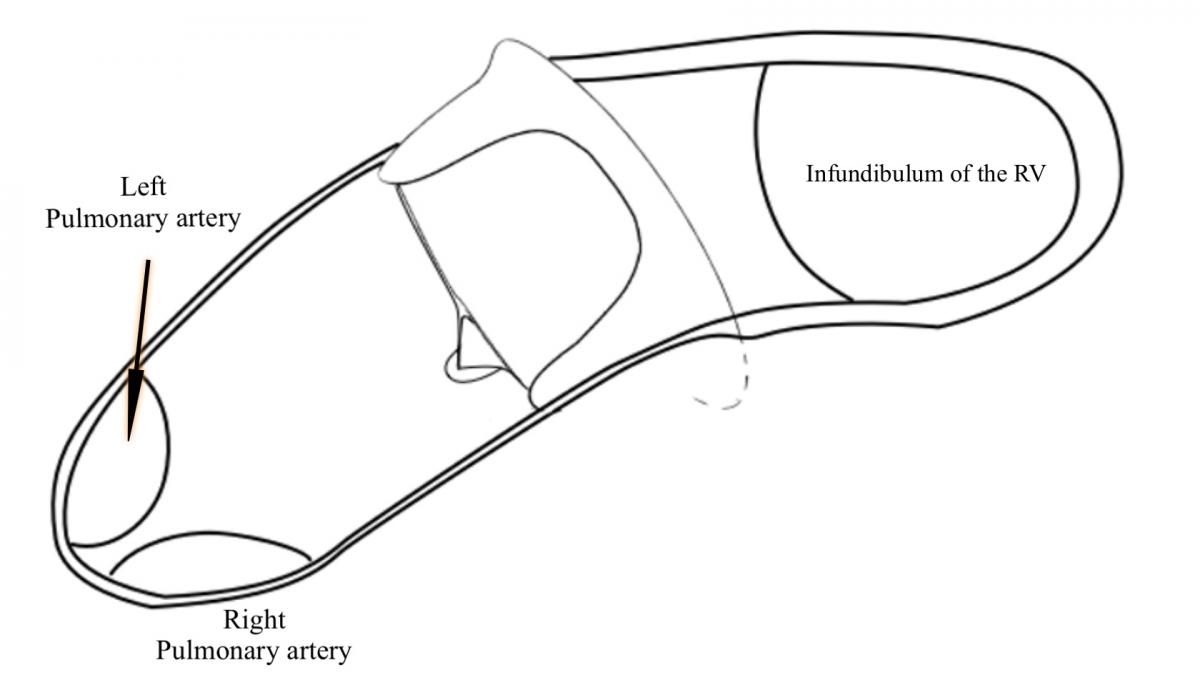
After the sternotomy the RVOT is dissected and the calcified transannular patch is excised. The appropriate size biological pulmonary valve is chosen according to the dimensions of the annulus. The valve is positioned with the two struts lying anteriorly (Diagram 1).
Diagram 1: The biological valve is positioned with the two struts anteriorly and the valve inclined inferiorly toward the PAs.
The angulation of the valve when lying in the main pulmonary artery is important, because it needs to be pointed inferiorly towards the pulmonary arteries (PAs). The valve will allow a good flow in this position, and this allows effective support of the transannular patch that will cover the valve. The valve struts will sustain the transannular patch without distortion, channeling the flow directly into the PAs.
The valve is secured with two hemi-continuous prolene sutures. This ensures the borders of the free edges of the RVOT are sutured at the level of the two anterior struts of the valve.
The transannular patch is measured using a silk string. The width of the patch is determined by the length of silk required to encircle the valve from the free edges of the RVOT at level of the annulus. The initial length of the patch needs to be long, as it will be customized precisely while being sewn.
The bovine pericardial patch is sutured to the pulmonary artery, and the suture line is carried at the level of the valve on both sides. Careful attention must be taken to prevent transannular leak at this level. The patch is folded and the ring of the valve is fixed to the transannular patch at the level of the fold. The patch is again folded back, trimmed to the dimensions of the RVOT, and sutured to the myocardium.
In some cases where the RVOT is extremely dilated, the valve can be sited directly into the ventricle. The valve is secured using the same technique. In this particular case, the transannular patch did not need to be folded.
Conclusion
In patients with free PR, implanting a competent valve associated with RVOT remodeling significantly reduces RV size early after operation. The authors’ results using the St. Jude Medical Trifecta pericardial valve for PVR are encouraging.