ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Thoracic Endovascular Aortic Repair (TEVAR)
Patient Selection
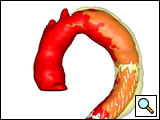
Indications for endovascular repair of the thoracic aortic aneurysm (Figure 1) are:
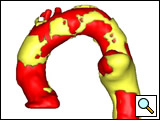
Figure 1: The three-dimension reconstruction (Medical Metrx Solutions, West Lebanon, NH) off the aortic-centerline from a contrast-enhanced high-resolution computer tomography scan showing an “ideal candidate” for TEVAR.
Patients with an asymptomatic descending aortic aneurysm larger than twice the size of the normal distal aortic arch in an orthogonal projection (at least 5 cm); and no evidence of connective tissue disorder. Symptomatic aneurysms mandate endovascular (or open repair), regardless of size. The patient should have proximal (distal to left common carotid) and distal neck of at least two cm without significant calcification or thrombus; the patient should have access vessels of at least 8 mm luminal diameter and without extreme tortuosity. Depending on device availability, the patient’s landing zone diameter has to be within the manufacturer’s instruction for use (IFU), e.g. for the Gore TAG device the proximal and distal landing zone should not be smaller than 23 mm or larger than 37 mm.
Operative Steps
- The patient is placed in a supine position. Both groins and the entire abdomen are prepped and draped. If the right radial/brachial artery needs to be accessed, the right arm is prepped and draped. TEVAR may be performed under general, regional or local anesthesia.
- Cerebrospinal fluid drainage is recommended for patients with previous abdominal aortic aneurysm repair, in whom iliac conduit or coverage of subclavian artery is planned. Furthermore, the patients with underlying thoracic aortic aneurysm requiring extensive stent graft coverage will require a prophylactic placement of the cerebrospinal fluid drainage catheter. Further discussion of the risk factors for paraplegia can be found in the reference section.
- The most common access site is the femoral artery (80% of the time); 10 mm conduit to common iliac artery is used 15% of time (figure 2); ascending aorta, distal abdominal aorta used 5% of the time. The right brachial/radial artery is accessed when planning on excluding any brachiocephalic vessels with the covered stent graft (Zone 0-2 deployment in the aortic arch). The details of iliac access will be discussed elsewhere within the Endoluminal Technology Portal.
A femoral artery cutdown is performed on the device access site. The patient is heparinized for a target activated clotting time ≥ 300 sec. This level of anticoagulation is maintained throughout the procedure, until the femoral artery cutdown is repaired and distal pulses are verified. A 12F sheath is used for femoral access to potentially accommodate a large diameter occlusion balloon if acute rupture occurs. An angled catheter and guidewire are used to access the abdominal aorta, and then advanced under fluoroscopic guidance into ascending aorta. The soft tip of the guidewire should reflect off the aortic valve, back into ascending aorta. It is important to keep this guidewire in place during the entire endovascular procedure. The anesthesiologist should be forewarned about these guidewires and watch for any arrhythmias they may cause.
- If there is a concern about aortic rupture or dissection, the angled catheter is exchanged for an intravascular ultrasound (IVUS) catheter. The IVUS and guidewire are advanced together, under direct IVUS imaging and fluoroscopy, into the ascending aorta. This is to keep the wire out of the contained perforation site or to keep the guidewire in the true lumen (in case of aortic dissection), respectively.
- The guidewire is then exchanged through the IVUS catheter for a stiff (soft tip) guidewire and the thoracic aorta interrogated with IVUS. The use of IVUS for thoracic procedures allows the surgeon to interrogate the thoracic arch and potentially map out (on the fluoroscopic screen) the arch vessels without the use of contrast. The device can be advanced and positioned in the arch and then the first angiogram performed to confirm branch vessel location, which may have changed with the device in place. The IVUS is also invaluable in dissections to assure the device is in the true lumen along the length.
- A percutaneous 6F sheath is placed in the contralateral femoral artery and a second guidewire positioned in ascending aorta. If the endoluminal device will be deployed distal to the subclavian artery (landing zone 3 & 4), a 6F pigtail catheter will be positioned in the ascending aorta. (If the left subclavian artery is going to be excluded with the covered device (landing zone 2), this guidewire will serve as a “bailout” access guidewire. If the device is deployed inadvertently over the left carotid artery, an 8mm x 8cm balloon will quickly be advanced over this guidewire and inflated across the proximal end of the device, to allow perfusion to the carotid artery orifice.)
- A percutaneous 4F sheath and subsequent pigtail catheter are placed in the right brachial artery when the left subclavian artery is to be excluded. The pigtail catheter is positioned in the ascending aorta.
- When dealing with a very tortuous aorta or an arch with a small radius, the body floss maneuver may be helpful in advancing the device through the arch. This is accomplished by advancing a 0.035 x 450cm hydrophilic guidewire from the right brachial artery to the femoral artery. The device may then be advanced over this guidewire.
- The pigtail catheter (transfemoral or through right brachial/radial) is used to perform an aortogram of the area of interest. After the angiogram is performed, the proximal neck is evaluated. The length and the diameter of the proximal and distal neck are therefore measured using the preoperative CT scan, intraoperative IVUS, as well as angiogram. The details of these imaging modalities will be discussed elsewhere within the Endoluminal Technology Portal. Based on these measurements, the stent graft(s) is (are) chosen, flushed with heparinized solution, and advanced into the proximal neck. A repeat angiogram is commonly performed to reconfirm the positioning of the device within aorta.
- Prior to device deployment, the systolic blood pressure is brought down to 100 mm Hg. If the patient is having a landing zone proximal to the left subclavian artery (zone 2 and higher), adenosine (36 mg for the first dose, 18 mg for subsequent doses) is administrated to gain a 4-5 sec cardiac arrest. The ventilator is stopped for the deployment in intubated patients. These two measures improve deployment accuracy by reducing dp/dt.
- After the deployment, the stent graft(s) are ballooned as suggested by IFU. An IVUS interrogation of the entire stent graft and surrounding aortic branches is performed. This will detect any circumferential stent mal-apposition to the proximal or distal landing zone that may lead to endoleak. A completion angiogram is performed to confirm lack of gross endoleak. However, a single aortogram may miss endoleak due to projection overlap. A bi-plane aortogram is more reliable in excluding any significant intra-procedural endoleak.
- After the completion angiogram, the introducer sheath is removed carefully leaving the wire in the aorta. If there is any concern about iliac artery injury, an iliac artery angiogram is performed. If no angiogram was performed, and the patient remains hemodynamically stable, an intact iliac artery can be assumed. In this case, the wire is removed, and the femoral artery is repaired in standard fashion. If an iliac artery conduit has been performed, there are three different ways to approach the closure, which will be addressed in another section within the Endoluminal Technology Section.
- The distal pulses are checked. If they correspond to preoperative baseline, the heparin is reversed. At this time, the sterile endovascular wires/catheters may be disposed.
Preference Card
Sheaths
- 12F sheath (Terumo 12fr x 10cm Pinnacle Sheath) is used for initial femoral artery access (to accommodate large diameter balloon in case of acute rupture, it will accommodate the IVUS as well)
- 6F sheath (Terumo 6fr x 10cm Pinnacle Sheath) used for percutaneous access of contralateral femoral artery or alternatively
- 4F sheath (Terumo 4fr x 10cm Pinnacle Sheath) used for right brachial/radial artery (for zone 0-2 deployment in the aortic arch)
- Micro-Puncture Introducer Set (Cook, Inc. 4fr, MPIS-401)
- 20-24F sheath as per stent graft manufacturer’s IFU
Catheters
- 5F Bern (Boston Scientific 5fr x 100cm Imager II Selective- Berenstein tip) or angled tip catheter used for initial femoral access
- 5F pigtail catheter (Cook, Inc. 5fr x 90cm Pigtail-Royal Flush Plus) used in the contralateral femoral artery (if brachial is not available)
- 4F Bern (Boston Scientific 4fr x 100cm Imager II Selective) or angled tip catheter (if brachial artery access is used)
- 4F pigtail catheter (Cook, Inc. 4fr x 90cm Pigtail-Royal Flush Plus) when using brachial artery
- 8.2F IVUS catheter (Volcano, 8.2fr x 90cm Vision PV, 8.2 fr, 8-12 MHz)
Guidewires
- 0.035 x 180cm Bentson Starter guidewire (Boston Scientific 0.035 x 180cm Bentson)
- 0.035 (or 0.025) x 260cm Stiff type guidewire with soft tip (Boston Scientific 0.025 x 260cm Platinum Plus ST Guidewire; Boston Scientific 0.035 x 260cm Amplatz Super Stiff Guidewire, 6 cm tip length; Boston Scientific 0.035 x 260cm Meier Guidewire, 10 cm tip length; Cook, Inc. 0.035 x 260cm TFE Coated Lunderquist Guidewire, 15 cm tip ; ev3, Inc. 0.035 x 260cm Nitrex wire)
- 0.035 x 180cm hydrophilic guidewire (Terumo 0.035 x 180cm Glidewire)
- 0.035 x 450cm hydrophilic guidewire (Terumo 0.035 x 450cm Glidewire)
Balloons
- 8mm x 8cm angioplasty balloon (Boston Scientific 8mm x 8cm Ultrathin Diamond Balloon)
- Large diameter (~ 40mm) compliant occlusion balloons (Medtronic 12fr Reliant Stent Graft Balloon, 46mm max diameter; Cook, Inc., 14fr CODA Balloon Catheter, 40mm max diameter)
Miscellaneous
- Dilator (Endovascular Dilator Set, Cook, Inc. 20-24 fr)
- Snare (Medical Device Technologies 27-45mm EN Snare)
- Luer-Lock (Boston Scientific FloSwitch HP)
- Needle for initial arterial access (Boston Scientific Arterial Entry Needle 18 gauge, 2 3/4in
- Torque device ( Terumo Torque Device)
Tips & Pitfalls
- The skills necessary to perform TEVAR can not be attained through short “courses”. A dedicated formal training (six months minimum!) is necessary in order to obtain the “wire skills” and to independently perform these endovascular procedures. The trained endovascular surgeon should be able to deal with intraoperative complications of TEVAR. Peripheral angioplasty/stenting should be part of the armamentarium of the endovascular surgeon. The current privileging criteria in most tertiary hospitals require a minimum of 100 diagnostic, 50 interventional and 10 TEVAR cases to become certified/privileged. This corresponds to the criteria suggested by the Society of Vascular Surgery.
- Most cardiothoracic surgeons will not meet the above training criteria (see also the credentialing section in the Endoluminal Technology Portal). In these cases, the necessary “wire skills” may be acquired by hiring a Cardiothoracic Surgeon who has had a dedicated training in endovascular surgery. Alternatively, collaboration with other physicians with interventional skills (vascular surgeons, interventional radiologist/cardiologist) will be crucial. In addition to this, one may consider having a mentor for the first five TEVAR cases.
- Train a dedicated nursing team for your endovascular cases. An Endosuite will substantially improve your imaging capabilities and broaden your endovascular options.
- Remember the PPPPP rule: Prior planning prevents poor performance! Preoperative imaging and its accurate interpretation is essential in preoperative planning. Look for poor access vessels, significant calcification or tortuosity in the iliac arteries. Have at least two backup plans including conversion to open repair with atriofemoral bypass with or without hypothermic circulatory arrest.
The patient will need to be followed up very closely in the postoperative period. A contrast CT with delayed non-contrast scan is performed at one month, three months, six months, one year and annually thereafter, to evaluate for endoleak, migration, device structural deterioration etc. The close follow-up (Figure 3) and the chance of postoperative issues requiring re-intervention has to be discussed with the patient as part of the informed consent.
References
- Schneider PA. Endovascular Skills, 3rd Ed. New York: Informa 2009.
- Khoynezhad A, et al. Risk factors of neurologic deficit after thoracic aortic endografting. Ann Thorac Surg 2007;83:S882-9.
- Makaroun MS, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg 2005;41:1-9.
- Manual of Peripheral Vascular Intervention. Casserly IP, et al. Philadelphia: Lippincott Williams and Wilkins; 2005.
- Mastery of Vascular and Endovascular Surgery. Zelenock GB, et al.; Philadelphia: Lippincott Williams and Wilkins; 2006.