ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Thoracoscopic Left Apical Trisegmentectomy
Increasing experience with thoracoscopic lobectomy has created an interest in performing anatomical segmentectomy using the thoracoscope, as it combines the benefits of lung conservation with avoidance of a thoracotomy. Typically a segment of the lung contributes 5% of the overall lung function, allowing for an improved postoperative FEV1 as compared to a lobectomy (1).
Segmentectomy was first described by Churchill and Belsey for bronchiectasis in 1939 (2). It was abandoned after Ginsberg demonstrated the superiority of lobectomy over sublobar resection in 1995 (3), but has returned as a lung cancer operation in recent years (4). The first description of thoracoscopic segmentectomy in an animal model was reported in 1997 (5). Thoracoscopic segmentectomy can be defined as an anatomical resection of a segment or segments of the lung with individual dissection, isolation, and transection of the vein, artery, and bronchus to the segment/segments using the thoracoscope (camera and monitor) for visualization, and dissection of structures with no rib-spreading (6). It is a challenging procedure. A clear and detailed knowledge of segmental lung anatomy and advanced thoracoscopic skills are required to perform it.
Patient Selection
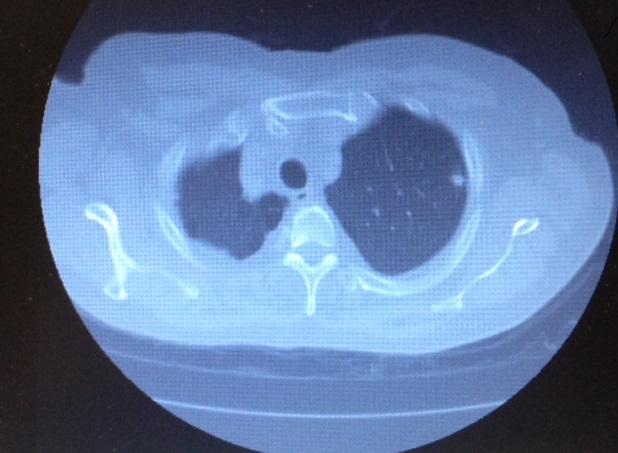
The left upper lobe has anatomically well-defined segments with a clear division of the hilar structures from the apical and lingular segments, allowing for an anatomical resection. The left apical tri-segments are the apico-posterior and the anterior segments of the upper lobe. These can be clearly isolated from the superior and inferior lingular segments, as the vasculature and airways are anatomically distinct from each other and can be clearly identified with surgical dissection. An alternate name for the operation is, therefore, lingular sparing left upper lobectomy (7). The lesion should be located well within the apical segments of the left upper lobe and be 1.5 cm or less in diameter. A CT scan is vital to aid in preoperative localization of the lesion and assessment of size and position within the lobe (Figure 1). Cross-sectional images, as well as coronal and sagittal imaging, should be used to confirm the position of the lesion, in addition to the vasculature and airway to the segments (6).
Figure 1: Anterior view of left hilum with phrenic nerve and pulmonary vein.
Left apical tri-segmentectomy may be performed for lung cancer, metastases to the lung, carcinoid, or bronchiectasis. Other reasons to perform a tri-segmentectomy are for a ground glass lesion, synchronous primaries, and to treat patients who have marginal lung function, are in advanced age, have poor cardiopulmonary reserve, and have received previous lung resection (6).
If there is doubt as to the location of a lesion within the segment to be resected, then segmentectomy should not be performed. This can be ascertained preoperatively by CT scan, or intraoperatively after the vessels and airway have been dissected, and visual and tactile assessment of the likely location of the lesion in relation to the segmental division can be made.
Routine preoperative assessment of lung function and effort tolerance should be made with calculation of postoperative FEV1, assessment of DLCO, and stair-climbing ability (3 flights) in order to assess suitability for lung resection. A V/Q scan can be performed to aid in the estimation of postoperative lung function, especially in patients who have limited cardiopulmonary reserve or previous lung resection (11).
Whether tri-segmentectomy should be performed for lesions with N1 disease within the segment, N2 disease at level 5 or 6, or in other N2 stations, is not clear. However, the current practice is to convert to lobectomy if N1 disease is discovered intra-operatively (8). In most patients with N2 disease, the N1 nodes are involved and lobectomy would appear to be indicated. In patients with skip metastases, the benefit of segmentectomy is unknown.
Operative Steps
The patient is placed on his/her side, with the left side up (as in a posterolateral thoracotomy position). Initially the table is rolled posteriorly, as this improves exposure of the anterior aspect of the hilum of the lung. Single lung ventilation is used. An arterial line and peripheral or central venous access should be performed depending on the individual patient.
The chest is prepped and draped as it is for a posterolateral thoracotomy. Intercostal nerve blocks with 0.5% marcaine are given in all spaces in which incisions are to be made. The surgeon stands anteriorly. The assistant is positioned either anteriorly next to the surgeon or posteriorly. The assistant operates the camera and uses the suction, or retracts the lung.
The thoracoscope is initially placed in the 7th or 8th intercostal space in the anterior axillary line. Using thoracoscopic visualization, a working incision is made in the 5th space or in line with the major fissure, between the mid and anterior axillary line. The skin incision is made just long enough to allow placement of a Weitlaner retractor. The intercostal incision is usually extended anteriorly and posteriorly beyond the skin incision to allow for 2-3 instruments to be used simultaneously through the working incision. A 3rd incision is made, usually in the 8th interspace, in line with the tip of the scapula or just anterior to it. Alternatively, this incision can be made using a thoracoscopic assessment of where it should be placed, depending on the angle needed to access the hilar structures with a stapler. Usually a combination of the above two techniques can be used to select the best site for the incision.
The camera is placed in the anterior incision, as this provides an excellent end-on view of the hilum. The camera can be rotated to provide the best view as the dissection proceeds (Figure 2). The upper lobe is grasped with a thoracoscopic ring clamp and retracted posteriorly, either through the working incision (by the surgeon) or via the posterior incision (by the assistant). A thoracoscopic straight sucker is placed through the posterior incision, or the working incision, to aid in the dissection of the tissues and to ensure a clear operative field.
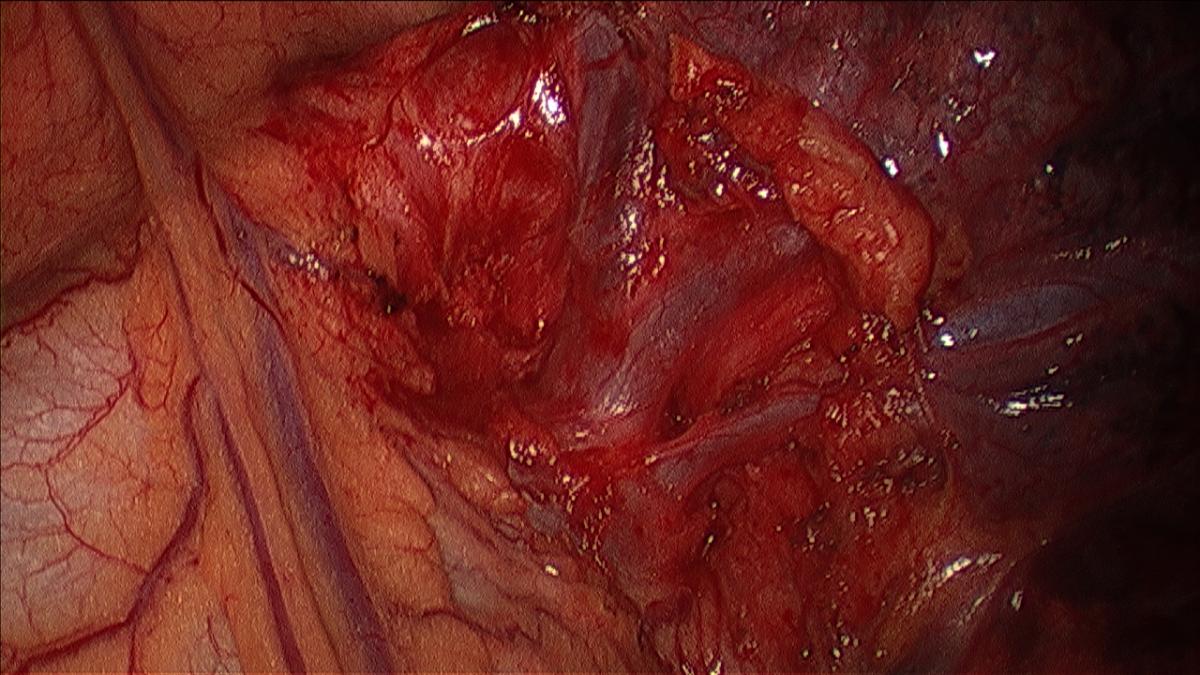
Figure 2: Dissected apical tri-segment and lingular vein.
After clearly identifying the course of the phrenic nerve, the mediastinal pleura overlying the anterior aspect of the superior pulmonary vein is opened sharply, in a vertical direction. Using the sucker, the pleura can be bluntly separated from the underling hilum and retracted to allow for division, either with harmonic scalpel or with cautery. The pleura is opened inferiorly until the inferior vein is identified, and superiorly over the main pulmonary artery to the level of the origin of the descending thoracic aorta. Cautery is not used in the concavity of the aortic arch to prevent injury to the recurrent laryngeal nerve.
The table is rolled anteriorly to allow improved exposure to the posterior hilum. The camera is switched to the posterior incision. The lower lobe is retracted superiorly using a ring clamp via the working incision, and the pulmonary ligament is divided with a harmonic scalpel after retracting the diaphragm inferiorly with the sucker via the anterior incision. The lower lobe is then retracted anteriorly with a thoracoscopic sponge stick (tonsil sponge in a thoracoscopic ring clamp) that is placed at the level of the lower lobe bronchus. Using the sucker, inserted through the opening in the mediastinal pleura created by dividing the pulmonary ligament, the pleura is retracted over the posterior aspect of the hilum and divided with a harmonic scalpel proceeding superiorly to the level of the origin of the descending thoracic aorta. As the dissection proceeds superiorly, the position of the sponge stick is also moved superiorly. The posterior aspect of the main pulmonary artery is dissected sharply and bluntly (sucker or peanut), clearing mediastinal soft tissue from the artery and sweeping the lung parenchyma anteriorly off the artery to expose the posterior aspect of the first or apico-posterior pulmonary arterial branch. This facilitates the circumferential dissection of the segmental arteries later in the dissection.
At this time confirmation that the mass is within the apical tri-segments should be made and the approximate line of division between the segments visualized.
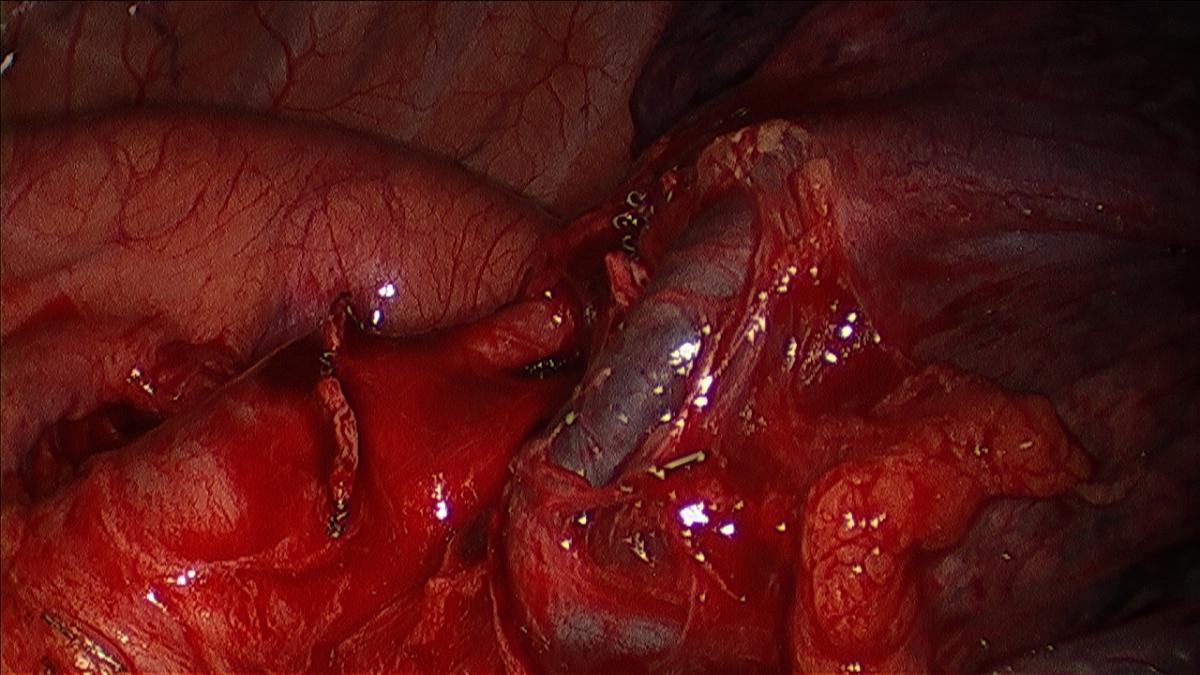
The camera is switched back to the anterior incision, the table rolled posteriorly, and the upper lobe retracted posteriorly. The superior pulmonary vein is dissected sharply and bluntly (sucker or peanut) circumferentially and distally until the trunk of the apical tri-segments and the lingular veins are clearly identified (Figure 3). Circumferential Isolation of the tri-segmental vein is done using a thoracoscopic right angle through the working incision, or by passage of a thoracoscopic curved vascular clamp via the posterior incision (Videos 1, 2).
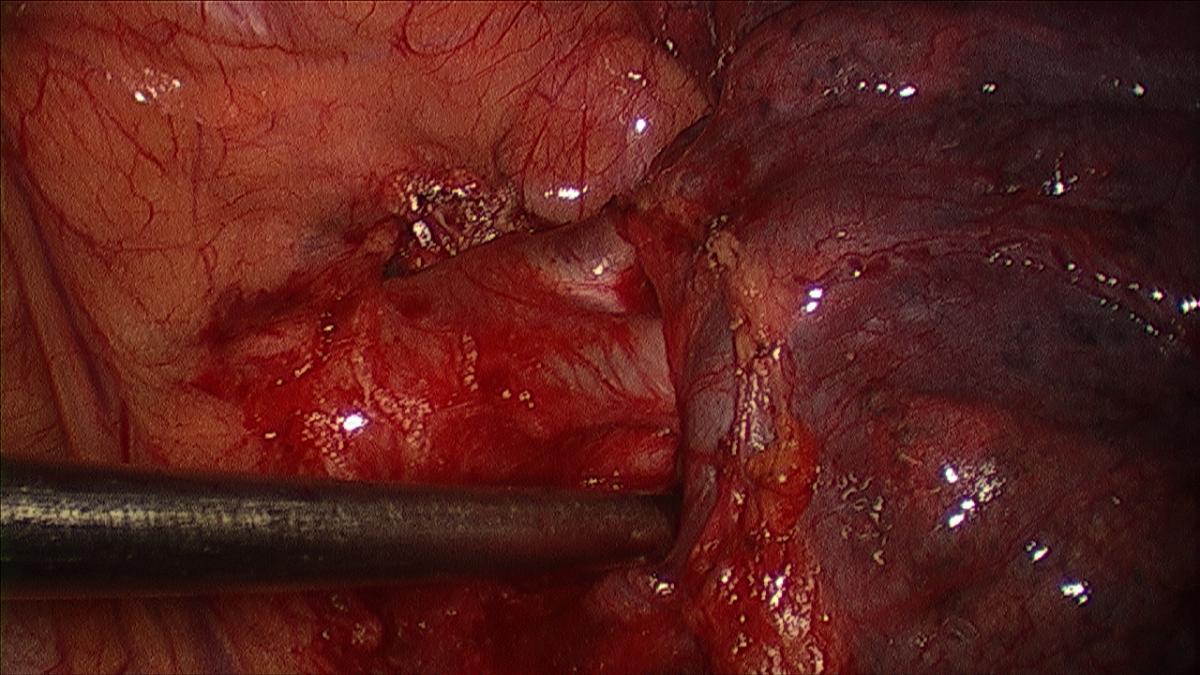
Figure 3: Dissected apical pulmonary arterial branches.
Video 1: Dissecting the apical tri-segmental vein.
Video 2: Dissected apical tri-segmental vein and lingular vein.
Often lymph nodes are encountered around the distal aspect of the superior vein, or at the confluence of the segmental veins into the superior vein. These should be sharply/bluntly dissected and removed, as by doing so the segmental veins are also dissected. This is best done by sharply incising the tissue around the node, allowing it to be held by a thoracoscopic ring clamp or retracted with a sucker, and then continuing the dissection sharply/bluntly or with harmonic scalpel until the node is completely freed from the surrounding tissue. These N1 lymph nodes should be subjected to frozen section analysis. If the lymph nodes are positive, a lobectomy should be performed.
The first pulmonary arterial branch to the left upper lobe, which is the artery to the apico-posterior segment, is dissected sharply and bluntly if adequate exposure is possible. The exposure of the artery is facilitated by retracting the superior vein inferiorly with the sucker (Figure 4, 5).
Any lymph nodes encountered are dissected, removed, and analyzed as described. If exposure is not adequate, the apical tri-segmental vein can be divided as described subsequently. Circumferential isolation of the artery is performed with a thoracoscopic right angle, or curved vascular clamp via the working incision (Videos 3, 4).
Video 3: Dissecting the first apical tri-segmental artery.
Video 4: Isolating the first apical tri-segmental artery.
The segmental vein is divided using an EndoGIA vascular stapler, either 30 mm or 45 mm in length, passed through the posterior incision. Prior to dividing the vein, the thoracoscopic curved vascular clamp is passed behind the vein to ensure that it is free and that the passage of the stapler around the vein will be smooth, without undue tension or retraction. The stapler is articulated so that the cartridge is at approximately 60-80 degrees to the long axis of the stapler, and the lung is retracted either anteriorly or posteriorly to allow for the smoothest passage of the stapler jaws around the vein. It is best to lead (pass posterior to the vein) with the thin limb of the stapler cartridge (Video 5).
Video 5: Dividing the apical tri-segmental vein.
Once the vein is divided, exposure of the apico-posterior arterial branch is improved and the artery is isolated (if it has not been done as previously outlined). The thoracoscopic vascular clamp is passed behind the arterial branch, either from the working incision or the posterior incision, depending on which incision provides the easiest route to the vessel. If the posterior aspect of the pulmonary artery has not been sufficiently dissected, it may be difficult to isolate the artery branch. Further dissection to release the posterior lung parenchyma can be performed sharply, either through the working incision or from a posterior approach (the lung is rolled forward with a sponge stick to expose the posterior aspect of the main pulmonary artery). The artery is then divided, as was described for the vein. The stapler can be passed from the posterior incision or via the working incision, using whichever allows the smoothest access to the artery (Video 6). The dissection is then continued along the main trunk of the pulmonary artery using both sharp and blunt dissection. This usually reveals another branch (anterior) of the pulmonary artery to the tri-segment, which is dissected, isolated, and divided as previously described (Figure 6; Video 7).
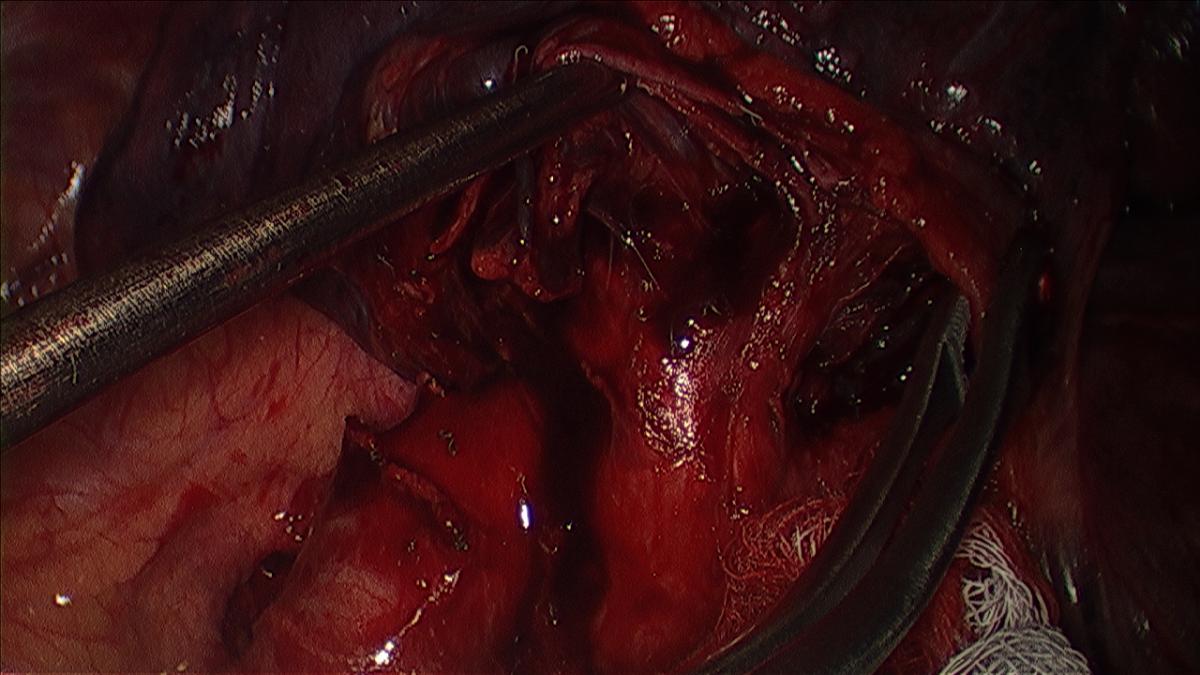
Figure 6: Dissected apical tri-segmental bronchus and lingular bronchus (under ring clamp).
Video 6: Dividing the first apical tri-segmental artery.
Video 7: Dividing the second apical tri-segmental artery.
At this stage, the bronchus is encountered and can be dissected sharply or bluntly depending on the state of the peribronchial tissues. It is easiest to identify the upper lobe bronchus posterior and inferior to the vein, but anterior to the plane of the pulmonary artery. Tracing this distally, the bifurcation of the bronchus into the apico-posterior and anterior trunks (upper tri-segments, Video 7) and lingular bronchus can be identified (Video 8). Lymph nodes are usually encountered around the bronchus, and their dissection and removal facilitate isolation of the bronchus. The upper tri-segmental bronchus will course superiorly, and can be circumferentially isolated after being dissected. It is easiest to use a thoracoscopic right angle to do this, but often the thoracoscopic sucker (with the suction occluded and passed from the posterior incision) is also a useful instrument. The thoracoscopic vascular clamp is now passed behind the bronchus from the posterior incision to ensure that the stapler will pass easily across the bronchus (Video 9). An EndoGIA 30 stapler (3.5 mm) is used to divide the bronchus, being passed through the posterior incision. The stapler is articulated to 60-80 degrees, as this offers the easiest angle for passage around the bronchus. The thin limb of the stapler cartridge is used to lead, passing behind the bronchus. At this time, it is important to ensure that with the bronchus occluded by closure of the stapler, the lingular portion of the upper and the lower lobe expand with ventilation. This ensures that only the segmental bronchus is divided. This also allows for an assessment of the tri-segmental margin in relation to the lingular segment, as the segment will remain relatively collapsed compared to the inflated lingula. The bronchus is then divided (Video 10). An alternative method is to inflate the lung and then clamp the bronchus, allowing the lung to deflate, which demarks the collapsed lingula from the inflated upper tri-segments.
Video 8: Dissecting the apical tri-segmental bronchus.
Video 9: Isolating the apical tri-segmental bronchus.
Video 10: Dividing the apical tri-segment bronchus.
The segment is separated from the remainder of the left upper lobe, conserving the lingula by dividing the lung parenchyma using an EndoGIA stapler. Compression of the lung parenchyma at the segmental margin with the thoracoscopic vascular clamp prior to placement of the stapler allows for an easier and more precise application of the same. Multiple firings of a 60mm 4.8 mm stapler are often needed. The stapler is usually passed from the working incision or the anterior incision with the articulation adjusted to be in line with the border of the upper tri-segments and the lingula (Video 11). The use of buttress material on the stapler should be determined based on the patient’s preoperative lung function, the appearance or quality of the lung parenchyma on the CAT scan, and by visual inspection.
The tri-segment is placed in an endo-bag and extracted through the working incision. It is easiest to move the tri-segment to the lower part of the chest cavity using a ring clamp, open the endo-bag in the upper part of the chest cavity where there is more space, and then place the segment into the endo-bag.
Video 11: Division of the apical tri-segment from the lingula.
Mediastinal lymph node sampling or dissection is performed. Hemostasis is secured, the chest cavity is irrigated, and .5% intercostal blocks are placed in all the incisions. Anterior and posterior 20Fr chest tubes are placed, under thoracoscopic vision, through the port sites used for the operation. The lung is re-expanded under vision to ensure proper orientation of the lingular segment. The bronchial stump can be examined using saline irrigation for an air leak. The working incision is closed in layers with 2-0 and 3-0 vicryl.
The patient is extubated. Chest tubes are used for suction overnight and are then removed.
Preference Card
- 30 degree thoracoscope
- Articulating EndoGIA stapler
- Thoracoscopic ring clamp/straight sucker/scissors/right angle/curved vascular clamp
- Harmonic scalpel
- Endobag
- 20Fr chest tubes
Tips and Pitfalls
- Have a clear and detailed knowledge of the segmental anatomy.
- Confirm that the lesion is well within the segment both preoperatively and intra-operatively.
- Dissect the hilar structures distally until the segmental anatomy is visualized.
- Clearly identify the structures before dividing any of them. If the anatomy is not clear, continue to dissect all the surrounding structures then irrigate, suction out the fluid, and use 4x4 gauze to further dry the field. Identify the proximal hilar structures first and then trace them distally to identify the segmental structures. If the segmental anatomy cannot be clearly identified, conversion to an open operation may be necessary.
- Identify as clearly as possible the parenchymal plane of separation of the apical tri-segments and the lingula, erring on the lingular side to ensure that an adequate margin is achieved.
Results
Anatomical segmentectomy has been recognized as an adequate operation for selected patients with Stage I lung cancer (1). In 2004 Houck et al. (7) reported on a small series of patients who had thoracoscopic left apical tri-segmentectomy or lingula sparing left upper lobectomy. They described the technique used to perform the operation and also outlined their patient selection for the operation (they selected Stage IA tumors that were in the apex of the lung and away from the lingual). The patients were staged with PET scan and had adequate pulmonary reserve to tolerate a lobectomy. 11 patients underwent thoracoscopic tri-segmentectomy and lymph node dissection with no conversions to open operation or need for transfusion. In ten patients, the chest tube was removed by postoperative day two. Median length of stay was three days, and there were no deaths. One patient developed atrial fibrillation, another had to have a chest tube replaced after it was inadvertently removed, and a third had to have completion lobectomy due to incorrect diagnosis on frozen section. Final pathology revealed Stage IA in seven patients, Stage IB in two, Stage IIA (microscopic N1 disease) in one, and Stage IIIA (microscopic N2 disease at level 5/6) in one . All patients were disease free at a mean follow up of 13.5 months. In their discussion, Houck and others compared the operation to an anatomical equivalent of a right upper lobectomy, and reserved its use for tumors well within the upper portion of the lobe and away from the lingula. They also suggested its use in patients with synchronous lesions in different lobes or limited pulmonary reserve. They concluded that the operation is feasible, safe to be performed thoracoscopically, and that long-term follow up is necessary to propose it as a standard for Stage IA tumors of the left upper lobe.
In 2007, Iwasaki et al (9) published their experience with left upper tri-segmentectomy (31 patients) comparing it to left upper lobectomy (55 patients) in patients with lung cancers of 2 cm or smaller. Of these, 20 had the procedure thoracoscopically and eight had a thoracoscopic segmentectomy. There was no difference between the groups in mortality and morbidity, including duration of chest tube drainage. Five-year survival was 69.7% in the segmentectomy group and 72.5% in the lobectomy group with no significant survival difference.
In a follow up to their initial series, Soukiasian et al. (10) reported a comparison between thoracoscopic left apical tri-segmentectomy and thoracoscopic lobectomy. 73 segmentectomies were compared to 226 lobectomies during a 12-year time period. 91% of the segmentectomies were done for primary lung cancer, with 68% of those for Stage IA tumors. The length of stay for segmentectomy was 3.8±3.3 days. The length of stay for lobectomy was 5.5±7.9 days. The overall complication rate was 37% for segmentectomy versus 17% for lobectomy, which was not statistically significant. Air leak as a complication occurred in 13.7% of segmentectomies and 4.9% of lobectomies, with atrial fibrillation occurring in 8.2% and 3.4%, respectively. There was one conversion to thoracotomy for segmentectomy. The incidence of pneumonia, subcutaneous emphysema, recurrent laryngeal nerve damage, pain, syncope, bradycardia, and wound infection were higher in the segmentectomy group. Bleeding, pneumothorax, readmission, empyema, bronchopleural fistula, myocardial infarction, and cerebrovascular accident were higher in the lobectomy group. In the discussion, the authors did not elaborate on a reason for the observed complications but mentioned that parenchymal transection during segmentectomy does not translate into longer hospital stay.
Perioperative mortality was 1.4% for segmentectomy and 0.8% for lobectomy. There was no statistically significant difference in survival between the two groups when compared to each other, or when broken down into Stage IA or B lung cancer. However, the Kaplan- Meier curves demonstrate a higher survival at five years for patients undergoing a segmentectomy. In summarizing their findings the authors state that thoracoscopic apical segmentectomy is equivalent to lobectomy in terms of mortality and morbidity, and that apical tri-segmentectomy provides survival similar to left upper lobectomy for Stage IA and IB lung cancer.
References
- Yang CF, D'Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg. 2012;94(2):668-81.
- Churchill ED, Belsey R. Segmental pneumonectomy in bronchiectasis: the lingula segment of the left upper lobe. Ann Surg. 1939;109(4):481-99.
- Ginsberg RJ, Rubenstein LV. Randomized trial of lobectomy versus limited resection for T1NO non small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615-23.
- Fan J, Wang L, Jiang GN, Gao W. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol. 2012;19(2):661-8.
- Koizumi K, Akaishi T, Wakabayashi A. Anatomic segmental resection of the lung by thoracoscopy: an experimental study. Surg Today. 1997;27(11):1051-5.
- D’Amico TA. Thoracoscopic segmentectomy : technical considerations and outcomes. Ann Thorac Surg. 2008;85(2):S716-S718.
- Houck WV, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery upper lobe trisegmentectomy for early-stage left apical lung cancer. Ann Thorac Surg. 2004;78(5):1858-1860.
- Swanson SJ. Segmentectomy for lung cancer. Semin Thorac Cardiovasc Surg. 2010;22(3):244-9.
- Iwasaki A, Hamanaka W, Hamada T, Hiratsuka M, Yamamoto S, Shiraishi T, Shirakusa T. Comparison between a case-matched analysis of left upper lobe trisegmentectomy and left upper lobectomy for small size lung cancer located in the upper division. Thorac Cardiov Surg. 2007;55:454–457.
- Soukiasian HJ, Hong E, McKenna RJ Jr. Video-assisted thoracoscopic trisegmentectomy and left upper lobectomy provide equivalent survivals for stage IA and IB lung cancer. J Thorac Cardiovasc Surg 2012;144:S23-6.
- Pate P, Tenholder MF, Griffin JP, Eastridge CE, Weiman DS. Preoperative assessment of the high-risk patient for lung resection. Ann Thorac Surg. 1996;61(5):1494-500.






Comments