ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Transapical Transcatheter Aortic Valve Implantation
Index
Transapical aortic valve implantation (TAVI) has become a feasible alternative to open techniques in high risk patients. Surgical technique and some critical tips and pitfalls are described.
Introduction
Aortic valve replacement (AVR) utilizing the open surgical techniques with the use of cardiopulmonary bypass (CPB) remains the standard of care for symptomatic patients with severe aortic stenosis (AS). Transcatheter aortic valve implantation (TAVI) has become a feasible alternative to open techniques in patients in whom open surgical treatment is contraindicated or those who are at high risk for perioperative morbidity and mortality. Many reports have shown the safety and efficacy of both transfemoral (TF) and transapical (TA) approaches to TAVI. However, a significant number of patients have poor vascular access due to either small size vessels or severe peripheral vascular disease and are only amenable to treatment with the TA approach.(1)
Patient Selection and Preparation
The currently available scoring systems for patients requiring AVR are flawed in that they are not applicable for a significant subset of those patients. They do, however, provide a framework for the complex decision making process for those who are at high risk for open surgical AVR including:
- STS score risk correlating with Mortality > 10%.
- Redo operation in an elderly patient in the presence of patent grafts that are at risk for injury due to close proximity to the chest wall based on coronary angiography and CT scan.
- Patient frailty, which is currently assessed subjectively by the surgeon but may also include factors such as six minute walk test.
- Previous history of radiation therapy with associated cardiomyopathy or severe radiation induced lung disease.
- Truly Porcelain aorta ideally determined by 3 D analysis of the ascending aorta and aortic arch that vascular control and aortic reconstruction would be difficult even with brief period of deep hypothermic circulatory arrest.
Once the patient is considered to have high surgical risk, he should undergo further workup including the following:
Cardiac catheterization to rule out significant coronary artery disease (CAD). PCI is recommended prior to TAVI if there is critical CAD that would not be better served with CABG. During cardiac catheterization also, attention should be directed at determination of the proper C-arm angles for fluoroscopic visualization of the aortic valve. This is typically a right anterior oblique and caudal view, or left anterior oblique and cranial view. It may be necessary to perform additional hemodynamic assessment of the aortic valve during this procedure as well. This is especially important in patients with left ventricular dysfunction and low gradient aortic stenosis by echocardiographic assessment. Additionally, it is important to assess the ilio-femoral vessels for tortousity and to confirm the severity of vascular occlusive disease.
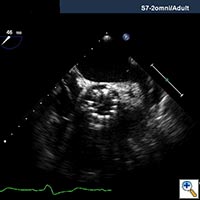
Echocardiography is important to confirm the severity of aortic stenosis, rule out bicuspid aortic valve (which a relative contra-indication) and to size the aortic annulus and left ventricular outflow tract (LVOT) (Figure 1). It is also necessary to assess for concomitant Mitral valve disease. Mild to moderate mitral regurgitation (MR) is frequently associated with AS in elderly patients but does not usually require any surgical intervention and does not represent a contraindication for TAVI. However, severe MR with bulky mitral annular calcification involving the LVOT has been associated with worse midterm outcome and increased risk of para-valvular leak (2).
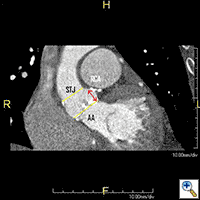
CT scan of the chest, abdomen and pelvis should be performed with three-dimensional reconstruction preferably with contrast if kidney function allows. The acquisition is performed with cardiac gating during the thoracic phase to assess the location of the coronary ostia in relation to the aortic annulus which is supposed to be > 10 mm to allow safe implantation of the valve (2). Additional assessment of the aorta CT scan and TEE are also important to delineate the morphology and measure the dimensions of the aortic root (Table, Figure 2).
|
Annulus at Hinge point |
Valve Size |
|---|---|
|
18-21mm |
23mm |
|
22-25mm |
26mm |
Additional assessment of the aorta and ilio-femoral vessels is performed to confirm poor access for transfemoral delivery and to prepare for a rescue cannulation in the event that conversion to cardiopulmonary bypass (CPB) becomes necessary during the TAVI procedure. Additional procedural planning performed at the time of three-dimensional CT assessment includes determination of fluoroscopic C-arm positioning and identification of left ventricular apex relationship to intercostal spaces. This assists with proper imaging and incision placement during the procedure.
Formal pulmonary function studies should also be performed to assess the severity of pulmonary disease, which so often coincides with aortic stenosis in high-risk patients. This is especially important when considering the transapical approach since these patients must be able to tolerate an anterior thoracotomy incision.
Operative Procedure
TAVI is usually performed in a hybrid operative suite designed and equipped to accommodate tools and personnel required for performing both open surgical and interventional procedures. TAVI procedures are best performed by a multi-disciplinary team including a cardiac surgeon, an interventional cardiologist and a cardiac anesthesiologist experienced in trans-esophageal echocardiogram (TEE). Other team members include a perfusionist, a surgical scrub nurse trained in transcatheter procedures, a circulating nurse, a radiology or catheterization laboratory technician and a surgical assistant. At least one member of the team must be trained in preparation and loading of the valve device, which is performed on the back table at the time of the procedure using a proprietary crimping device.
The procedural steps are as follows:
- 5 F femoral arterial sheath is inserted in the femoral artery with the least calcification and tortousity. This sheath is used to insert the pigtail catheter to be used for aortic root injections and for possible arterial cannulation in the event rescue CPB is needed.
- 5 or 6 F femoral vein sheath is also inserted for transvenous pacemaker insertion and as a venous access site for venous cannulation in case CPB is required in emergency situations. Some surgeons prefer direct placement of pericardial wires through the thoracotomy incision.
- Concurrently, anterolateral mini-thoracotomy is performed in the fifth or sixth intercostal space to obtain a straight access to the left ventricular apex. This is best determined by three-dimensional assessment of the preoperative CT scan of the chest.
- Pericardiotomy is performed over the left ventricular apex and the heparin is administered at 100U/ kg to achieve an ACT > 300 seconds.
- Two concentric purse string sutures reinforced with pledgets are placed at the left ventricular (LV) apex. We prefer to place two deep additional mattress sutures at this site.
- The valve is loaded on the delivery system and checked for proper orientation and seating. For balloon expandable devices, preparation will include crimping the valve over the balloon in a sterile fashion using a special machine designed for this purpose.
- The LV apex is punctured right at the center of the purse string sutures with a standard access needle.
- Soft access wire is inserted in the LV through the needle and directed to the AV then a 12F sheath is delivered into the LV apex to maintain intraventricular access. The Berman pulmonary artery (PA) catheter is then floated through the LV and across the AV, around the aortic arch all the way down to the abdominal aorta.
- A stiff Amplatz wire (260 cm) is then introduced through the catheter.
- The 26 F introducer sheath is delivered over the Amplatz wire through the LV apex.
- Next, Aortic balloon valvuloplasty is performed using a size 20 mm X 5 cm balloon during a brief episode of rapid ventricular pacing (RVP).(4)
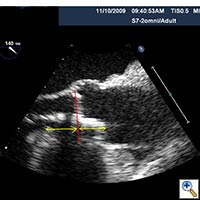
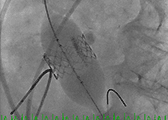
- The Sapien valve is then delivered through the introducer sheath and positioned across the aortic annulus with 50% of the valve above the annulus and 50% below the annulus. Both TEE and aortic root injection are used to confirm the position of the valve and its spatial orientation. Ideally, the valve should be in line with the long axis of the ascending aorta and perpendicular to the aortic annulus (Figure 3, 4, 5).
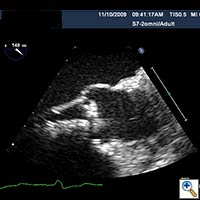
Figure 3. Direct echocardiographic visualization of the left ventricular outflow tract during valve insertion.
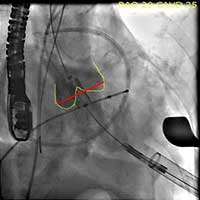
- The valve is deployed in position during direct fluoroscopic visualization (Figure 6) with another brief period of rapid ventricular pacing to reduce ejection. Respirations are also suspended during this period to limit the movement within the thoracic cavity and optimize visualization. Clear communication is critical during this brief period of the procedure and so one person commands the timing of each step of the procedure in a standard clear format.
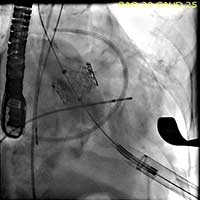
Figure 6. The valve is deployed in position during direct fluoroscopic visualization with a brief period of rapid ventricular pacing to reduce ejection.
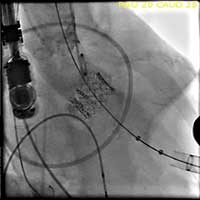
- After deployment, both TEE and another angiographic root injection are performed to confirm the position of the valve (Figure 7, 8), rule out paravalvular or central leak and to confirm the patency of the coronary arteries. Occasionally, the valve may require an additional insufflation with additional saline.
- Once the result is satisfactory, the introducer sheath is removed and the purse string sutures are tied to secure hemostasis.
- Protamine is administered and a left pleural chest tube is inserted.
- Routine closure of the access site incision is performed.
Preference Card
- Routine Open cardiac surgical set for AVR.
- Sheaths:
- 5F, 6F, and 12F sheath (Terumo Co. Somerset, NJ USA).
- 26 F introducer sheath (Edwards Lifesciences, Inc., Irvine, California)
- Catheters:
- 5F pigtail catheter (Cordis Corporation, Johnson Johnson, Warren, NJ, USA).
- Berman PA catheter
- Guidewires:
- 0.032” x 145 cm Schneider J-Tip Guide Wire (Boston Scientific Corporation, Natick, MA USA).
- AMPLATZ Super-Stiff 0.035” x 260 cm length x 6 cm Flexible Tip Length (Boston Scientific Corporation, Natick, MA, USA).
- Balloon:
- 20 mm X 5 cm (Z med Numed).
- Temporary pacer wires:
- Bipolar pacer wires (Medtronic)
Tips & Pitfalls
LV Apical Access
The apex is preferable to the free wall due to greater convexity and reduced wall stress. Select an area free of epicardial fat, take full-thickness bites in the myocardium and gain access with a needle precisely at the central point within the sutures to reduce the likelihood of extending the enlarging myocardial defect beyond the control sutures. Avoid severe hypertension especially when tying the sutures. Occasionally the sutures are tied during a period of rapid ventricular pacing.
If Cardiac Arrest Occurs
Do not hesitate to initiate temporary femoro-femoral cardiopulmonary support or to proceed for sternotomy if there is no sign of improvement with initial resuscitation. If chest compression is required during resuscitation, stent positioning and expansion of the valve must be reevaluated after resuscitation as the device may get crushed or displaced.
Avoiding mechanical complications:
- The most critical part of the procedure is proper positioning. If the valve is too deep in the ventricle (> 50 %), there is a risk of embolization into the left ventricle or inadequate closure of the valve. If the valve is too shallow (< 50 %), there is a risk of aortic embolization, coronary occlusion or excessive para-valvular leak.
- Avoid aggressive over dilation of the aortic annulus and valve over sizing in patients with small heavily calcified aortic annulus and aortic root to avoid rupture of the aortic annulus and almost certain death.
- Avoid aggressive over sizing deployment of the valve too far below the aortic annulus in patients with small LV outflow tract to decrease the risk of AV block.
- When a guide wire is advanced into the left atrium, the wire should be completely withdrawn into a delivery sheath before trying to reposition it to avoid tangling between the MV chordae. If the wire forms an unusual angle before entering the aortic valve, it may suggest that the wire likely passes through and is distorted by the mitral chordae before reaching the aortic valve. We have routinely used Bernan pediatric PA catheter to cross the valve to avoid the mitral sub-valvular apparatus.
- Always keep the guide wire in place and never lose access till the end of the procedure and after confirming the position of the valve and its stability. If the valve embolizes distally, anchor it to the aorta preferably distal to the origins of arch vessels to avoid compromise of blood flow through the arch vessels.
- If a bulky calcium load or a heavily calcified cusp is seen, it may be safer to leave a wire in the coronary arteries before aortic valvuloplasty is performed to allow recanalization if embolization occurs (5).
- If moderate to severe central or paravalvular leak occurs, the balloon can be reinflated to release the leaflets in case it is still crimpled or alternatively a valve in valve implantation can be performed.
- In patients with Porcelain aorta, beware of those with a very small and calcified sino-tubular junction as this may impair balloon insufflations and cause “ Water Mellon” seeding of the valve and device backwards into the ventricle.
Postoperative Care
Routine care as for conventional AVR with special attention to the following points:
- Early extubation.
- Close monitoring of renal function and adequate hydration to avoid contrast injury to the kidney.
- Keeping the temporary pacer wires for additional days in those patients who may be at risk of late atrio-ventricular (AV) conduction block.
Complications
Intra operative Complications:
- Apical laceration and hemorrhage
- Cardiac arrest
- Rupture of aortic root or annulus
- Heart block and arrhythmia
- Myocardial Ischemia or Infarction
- Mitral valve apparatus injury and worsening of mitral regurgitation
- Valve migration and embolization
- Coronary ostial obstruction
- Paravalvular or central leak
Postoperative Complications:
- Bleeding requiring re-exploration of the chest
- Acute renal failure
- Thrombo-embolic events and stroke
- Left Ventricular pseudoaneurysm
Results
To date, the only randomized controlled trial on TAVI is the PARTNER trial. The trial has two arms; the first is comparing medical therapy to transfemoral TAVI in patients who are not candidate for open surgical intervention. The second arm is comparing TAVI (either transfemoral or transapical) to open surgical therapy in patients who are considered to have very high risk for open surgical treatment. The trial just finished enrolling patients and the results are not available yet.
There are a number of non-randomized studies that showed a 30-day mortality of 10 % with 75% survival at one year (6), which is comparable to open surgical results (7).
References
- Kurra V, Schoenhagen P, Roselli EE, Kapadia SR, Tuzcu EM, Greenberg R, et al. Prevalence of significant peripheral artery disease in patients evaluated for percutaneous aortic valve insertion: Preprocedural assessment with multidetector computed tomography. J Thorac Cardiovasc Surg. 2009 May;137(5):1258-64.
- Masson JB, Kovac J, Schuler G, Yen J, Cheung A, Kapadia S, et al. Transcatheter aortic valve implantation: review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc Interv. 2009;2(9):811-20.
- Akhtar M, Tuzcu EM, Kapadia SR, Svensson LG, Greenberg RK, Roselli EE, et al. Aortic root morphology in patients undergoing percutaneous aortic valve replacement: evidence of aortic root remodeling. J Thorac Cardiovasc Surg. 2009 Apr;137(4):950-6. Epub 2009 Feb 23.
- Moon MC, Dowdall JF, Roselli EE. The use of right ventricular pacing to facilitate stent graft deployment in the distal aortic arch. J Vasc Surg. 2008 Mar;47(3):629-31.
- Kapadia SR, Svensson L, Tuzcu EM. Successful percutaneous management of left main trunck occlusion during percutaneous aortic valve replacement. Catheter Cardiovasc Interv. 2009;1; 73: 966-72.
- Svensson LG, Dewey T, Kapadia S, Roselli EE, Stewart A, Williams M, et al. United States feasibility study of transcatheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg. 2008;86:46–54.
- Walther T, Schuler G, Borger MA, Kempfert J, Seeburger J, Rückert Y, et al. Transapical aortic valve implantation in 100 consecutive patients: comparison to propensity-matched conventional aortic valve replacement. Eur Heart J. 2010 Mar 16. [Epub ahead of print]