ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Acquired Left Ventricle to Right Atrial Shunt (Gerbode Defect) and Massive Pulmonary Embolus
Introduction
Dr. Gerbode first described a left ventricle (LV) to right atrial (RA) shunt in 1958. These defects are usually congenital, but cases of acquired defects secondary to aortic or tricuspid valve endocarditis have been described. The acquired defect differs from the congenital defect in that it is a direct communication between the left ventricle and right atrium. We report a 7-year-old girl who suffered from acute bacterial endocarditis resulting in a Gerbode defect, involvement of three valves, and a thrombus occluding the left pulmonary artery. She was successfully treated with autologous pericardial patch closure of her left ventricle-to-right atrial connection, tricuspid valve repair, mitral and aortic valve debridement, and a left pulmonary artery thromboendarterectomy and embolectomy. The patient was discharged home and returned to school in good clinical condition.
Case Presentation
A seven-year-old girl presented to the emergency room (ER) with a six-day history of nausea, vomiting, and fevers. She has a history of aortic coarctation repair at 6 months of age. Symptomatically, she did well after that surgery, but had been followed for a residual coarctation. She presented to the ER hypotensive and was given three boluses of fluid and subsequently started on dopamine and transferred to the Pediatric Intensive Care Unit. Her C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were elevated. Her physical exam revealed a V/VI holosystolic ejection murmur heard over the entire sternal border and diffuse crackles. An electrocardiogram revealed sinus tachycardia without heart block. Her blood culture on admission grew methacillin sensitive Staphylococcus aureus. She was started on Vancomycin and Cefotaxime. Cardiology was consulted secondary to her history of congenital heart disease and the possibility of endocarditis. An echocardiogram revealed a large mass in the RA located superior to the tricuspid valve septal leaflet and moderate to severe tricuspid valve regurgitation (TR). There was question of a left to right shunt from either the LV or aorta to the RA. A computed tomographic (CT) angiogram of the chest demonstrated there was a connection between the LV and RA, minimal residual coarctation, and an embolus occluding the left pulmonary artery (PA) (Figures 1, 2a, and 2b). She had persistent bacteremia and septicemia despite antibiotics, with progressive pulmonary edema. Less than 24 hours after cardiology was consulted, the patient was emergently taken to the operating room with the diagnosis of an acquired Gerbode defect and pulmonary embolism.
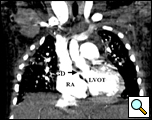
Figure 1. Computed tomographic scan showing left ventricle to right atrial shunt (arrow)
RA= right atrium; LVOT= left ventricular outflow tract; GD = Gerbode Defect
Figure 2a. Computed tomographic scan showing left pulmonary artery embolus (arrow)
PE= pulmonary embolus
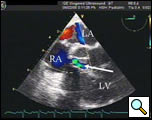
Preoperative transesophageal echocardiography (TEE) reconfirmed the preoperative diagnosis of a large LV-to-RA shunt, a large RA mass, and moderate to severe TR (Figures 3 and Video 1). Using cardiopulmonary bypass, she was cooled down to 24°C and cardiac arrest was achieved with antegrade cardioplegia. Externally, we could see that the left PA before the first branch was filled with a mass and was discolored. Through a right atriotomy, a large 2 cm x 1.5 cm vegetation in the RA, which served as a windsock-type tunnel for the LV-to-RA shunt, was identified (Figure 4a). This windsock connection was located right above the tricuspid valve and encroached on the base of the commissure between the septal and anterior leaflets, directly in the area of the conduction system. There were drop vegetations on the tricuspid valve besides the encroaching mass, all of which were causing tricuspid regurgitation. The aorta was then opened and the aortic valve was examined. The defect in the LV was in the intra-trigonal area between the right coronary and noncoronary cusps of the aortic valve (Figure 4b). There were also several drop vegetations on the aortic valve as well as on the mitral valve chordae. These vegetations were resected. The windsock-type mass in the right atrium was resected revealing a one centimeter defect from the RA into the LV just above the tricuspid valve annulus. This defect represented a type of acquired Gerbode defect. We were quite surprised that the conduction system was not compromised preoperatively since the defect eroded into the apex of the triangle of Koch.
Video 1
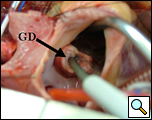
Figure 4a. Windsock mass in right atrium with probe through right atrial side of Gerbode defect (arrow)
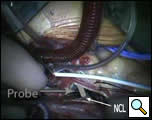
Figure 4b. Probe through Gerbode defect seen entering the left ventricle (View Through Aortic Valve)
NCL: non-coronary leaflet of the aortic valve; GD=Gerbode defect
Probe has been colored to allow better visualization.
The inferior rim of the membranous septum was intact, although encroached upon by the inflammatory process. Since the defect had no depth with the windsock resected, we felt comfortable closing this defect using one pericardial patch on the RA side. Assessing the defect from both sides, we felt there would be less of a chance of causing complete heart block putting a patch from the right atrial side. Prolene sutures in a horizontal mattress fashion were used to attach an autologous pericardial patch to the RA side of this defect. The posterior medial sutures were placed underneath the tricuspid valve with the pledgets on the ventricular side of the valve, then through the leaflets and then the patch. This was the only way to affix the patch in this area and to recreate the TV annulus, since there was no other available tissue besides leaflet tissue (Video 2). A DeVega annuloplasty
repair was also performed. A vertical incision on the left PA revealed total blockage of the left PA before its first branch, with what appeared to be chronic thrombus as well as fresh thrombus. We performed a thromboendarterectomy of the left PA and its primary branches as well as an embolectomy with a Fogarty balloon catheter (Figure 5). The right PA did not have any identifiable thrombus or emboli. Through the septum primum, the mitral valve was seen to have vegetations on both the anterior and posterior leaflets, which were resected. The patient was weaned from cardiopulmonary bypass without difficulty and in normal sinus rhythm. Postoperative TEE demonstrated minimal tricuspid regurgitation, trivial aortic insufficiency, and no residual LV-to-RA shunting, vegetations, nor mitral regurgitation. The patient was hemodynamically stable on epinephrine and Vasopressin with good hemodynamics, ventilation, and oxygenation.
After discharge from the ICU on POD 7, her course was unremarkable except for some myelosuppression secondary to nafacillin and a minor gastrointestinal bleed secondary to her anticoagulation therapy for her pulmonary embolus. Her echocardiogram prior to discharge demonstrated no intracardiac vegetations or intracardiac shunts and none to trivial valvar insufficiency. She was discharged home on 3-6 months of antibiotic suppressive therapy and has returned back to school.
Discussion
A Gerbode defect is a LV–RA communication, however since the original description in five patients by Gerbode et al., further study and classification of the defect reveals two distinct types [1].
Figure 6. Schematic demonstrating the two types of Gerbode defects. arrow (A) : acquired type; arrow (B): congenital type RA = right atrium; RV = right ventricle; LA = left atrium; LV = left ventricle; Ao = aorta; TV = tricuspid valve; IVS = interventricular septum; MV = mitral valve
Riemenschneider and Moss have described two types of LV-RA communication based on whether the defect is a direct LV-to-RA communication or indirect [2]. The acquired defect occurs between the LV and the RA through the superior aspect of the membranous (atrioventricular) septum above the insertion of the tricuspid valve in the membranous septum (Figure 6). These defects are usually secondary to endocarditis but have been described after operative trauma or ischemic heart disease [3,4]. The more common congenital type is a communication between the LV and the RA through a defect in the interventricular septum and the septal leaflet of the tricuspid valve. Therefore, unlike the acquired defect this is an indirect LV-to-RA shunt since the blood first travels between the left and right ventricle below the tricuspid valve and then is quickly shunted through a defect (i.e. cleft) in the TV to the RA, producing the physiologic effect of an LV-to-RA shunt (Figure 6). The basic anatomical defect though is a ventricular septal defect associated with a TV defect. Our patient’s defect was an acquired defect.
Preoperative diagnosis of an LV-RA communication is often very difficult. TEE has been demonstrated to be superior to trans-thoracic echocardiography (TTE) in identifying not only vegetations and valvar pathology, but also associated complications of endocarditis such as abscess and fistula formation [5]. In our patient, the preoperative diagnosis of a vegetation and an LV-to-RA communication was suspected by TTE, however the vegetation in the right atrium was not the typical oscillating type but rather a fixed mass just above the TV. This appearance can be typical for an aortic or ventricular to atrial shunt as echogenic debris builds up in this area of turbulent flow creating a windsock, as seen here (Figure 4a). Since the diagnosis remained unclear, a CT was performed primarily to assess the prior coarctation repair, but also we hoped it would better define the possible intracardiac shunt. The CT clearly identified the LV-to-RA shunt but also revealed a large pulmonary artery embolus (Figures 1-2). CT scan and MRI are diagnostic options in addition to echocardiogram in this disease process, especially as the speed at which one can obtain and perform a cardiac MRI continues to improve.
In conclusion, this 7-year-old girl suffered from an acquired Gerbode defect and pulmonary thromboembolus secondary to acute Staphylococcus aureus endocarditis and was successfully treated with autologous pericardial patch closure of the left ventricle-to-right atrial connection, tricuspid valve repair, and left pulmonary artery thromboendarterectomy and embolectomy.
Video 2
References
- Gerbode F, Hultgren H, Melrose D, and Osborn J. Syndrome of left ventricular-right atrial shunt. Successful surgical repair of defect in five cases, with observation of bradycardia on closure. Ann Surg 1958;148:433-46.
- Riemenschneider TA and Moss AJ. Left ventricular-right atrial communication. Am J Cardiol 1967;19:710-8.
- Velebit V, Schoneberger A, Ciarnoni S, et al. Acquired left ventricular-to right atrial shunt (Gerbode defect) after bacterial endocarditis. Tex Heart Inst J 1995;22:100-2.
- Alphonso N, Dhital K, Chambers J, and Shabbo F. Gerbode’s defect resulting from infective endocarditis. Eur J Cardiothorac Surg 2003;23:844-6.
- Elian D, Di Segni E, Kaplinsky E, Mohr R, and Vered Z. Acquired left ventricular- right atrial communication caused by infective endocarditis detected by transesophageal echocardiography: case report and review of the literature. J Am Soc Echocardiogr 1995;8:108-10.