ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Case Report: Do Hepatic Artery Infusion Pumps Cause Recurrent Pleural Effusions?
Harris E, Castillon G. Case Report: Do Hepatic Artery Infusion Pumps Cause Recurrent Pleural Effusions?. March 2020. doi:10.25373/ctsnet.11977470
Introduction/Background
Hepatic artery infusion pump chemotherapy (HAIPC) for colorectal liver metastasis (CRLM) is a relatively new technique. Its side effects are not well studied, owing to its novelty. The purpose of this paper is to further understand the side effect profile of HAIPC as it relates to recurrent pleural effusions in this patient population. This case report describes a 48-year-old man with CRLM being treated with HAIPC, who presented with recurrent pleural effusions found to be benign/transudative after right video-assisted thoracoscopic surgery. This case suggests that HAIPC may cause recurrent pleural effusions as a side effect. Furthermore, the authors hypothesize that perihepatic inflammation causing a sympathetic pleural effusion could be a sign of hepatic toxicity from HAIPC, ranging from hepatitis to primary biliary sclerosis. Lastly, the authors aim to guide the differential diagnosis of pleural effusions in the cancer patient being treated with HAIPC.
In suspected patients with colorectal cancer, the liver is the most common site of metastasis, with 15% of patients presenting with metastatic disease at the time of diagnosis and up to 60% of patients developing liver metastases during the course of their disease (1-3). In colorectal cancer patients with liver only or liver dominant metastatic disease who have progressed on first line chemotherapy, HAIPC combined with systemic agents is a viable therapeutic option wherein the HAIPC demonstrates an improved liver tumor response rate compared to systemic therapy alone (43% vs 18%) (2). This success is owed to the fact that CRLM receive their blood supply predominantly through the hepatic artery, and therefore this technique provides high intratumoral chemotherapy concentrations (3). In this case report, the authors hypothesize that the local toxicity and inflammatory effects of the HAIPC could manifest as a subclinical hepatitis and stimulate a sympathetic pleural effusion.
Case Description
A 48-year-old man with a history of metastatic rectal cancer to the liver status post folfox and bevacizumab, proctosigmoidectomy, coloanal anastomosis, loop ileostomy, hepatectomy segment VI, hepatic ablation of segments V and IVb, and hepatic artery infusion pump placement with FUDR. Seven months after pump placement, thoracic surgery was consulted regarding a recurrent right pleural effusion (Image 1).
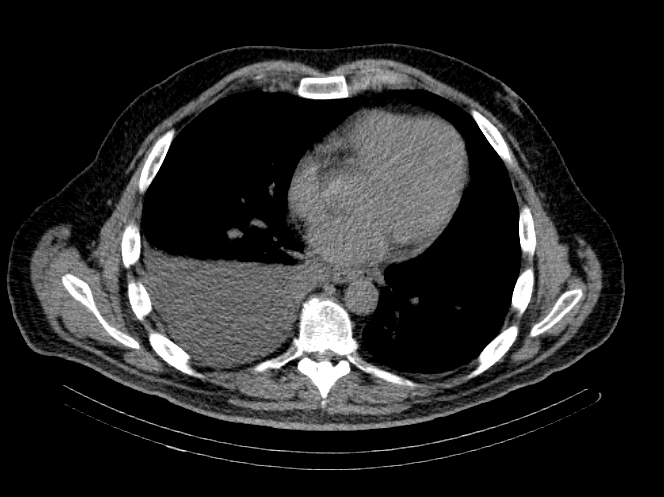
Image 1: CT Chest
IMPRESSION: Moderate to large right-sided pleural effusion.
The only symptom the patient endorsed was right-sided subscapular pain which had been present for four months. This pain was temporally related to use of his infusion pump; the pain improved with the chemotherapy infusion but worsened with heparinized saline infusion. The patient’s last dose of HAIPC with FUDR was performed one month prior to the consultation.
|
Triglycerides |
Protein g/dl |
pH | Lipase Unit/L |
LDH Unit/L |
Amylase Units/L |
Albumin g/dL |
Glucose mg/dL |
Culture Aerobic w/ gram stain |
Culture |
Fungus | |
| 3 mos preop |
42 | 4.9 | 7.40 | 10 | 127 | 15 | 3.1 | 119 | Negative | Negative | |
| 1 mon preop |
51 | 4.6 | 7.61 | 100 | 2.7 | 97 | Negative | Negative | Negative | ||
| Prior to surgery | 55 | 4.5 | 116 | 111 | Negative, rare wbc |
Negative | Negative |
Table 1. Transudative Pathology
The patient notably had undergone two right thoracenteses demonstrating transudative fluid characteristics (Table 1). The patient had multiple interrogations of his hepatic artery infusion pump, including a nuclear medicine hepatic pump with SPECT/CT (Image 2) and an IR venogram (Image 3), demonstrating adequate position and functioning of the hepatic artery infusion pump with appropriate perfusion of the liver and no evidence of extrahepatic radiotracer. The patient’s labs preoperatively were all within normal limits, including normal biliary labs with AST/ALT 23/15, AP 55, and total bilirubin .3.
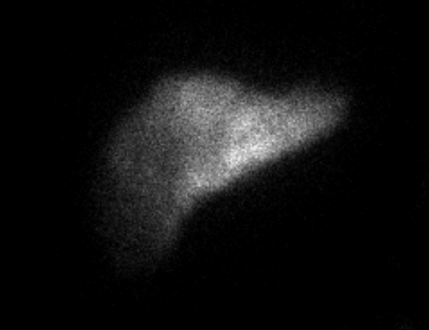
Image 2. IR Procedure: NUC MED HEPATIC PUMP with SPECT/CT
IMPRESSION: Appropriate perfusion of the liver after injection of radiotracer into the hepatic arterial infusion pump. No evidence of extrahepatic radiotracer collection identified.
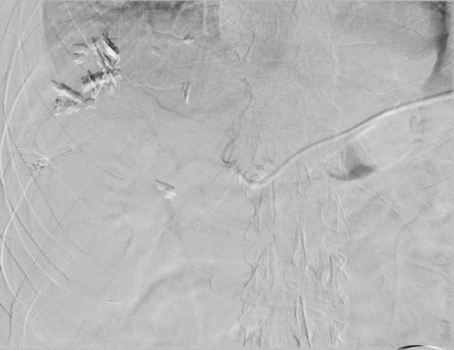
Image 3. Procedure - IR venogram PROCEDURE: Hepatic Arterial Infusion Pump Evaluation, MAA Infusion
An angiogram was performed via the pump showing opacification of both right and left hepatic arteries. The MAA was infused. The pump was flushed with heparinized saline. The needle was removed.
The patient underwent a right VATS pleural biopsy, pleural fluid cytologic analysis, and PleurX catheter placement. At operation, approximately 600 cc of amber clear pleural fluid was present, there was no evidence of pleural implants, and several tan-looking areas were noted on the parietal pleura which were biopsied. Frozen pathology was negative for malignancy, and final pathology demonstrated reactive mesothelial cells with chronic and minimal acute inflammation (Pathology 1). The postoperative pleural fluid analysis was consistent with transudative pathology (Table 1).
Pathology:
Path 1- Surgical Pathology
Right parietal pleural biopsy, irregular tan pink soft tissue, 1.2 1.1 x .3 cm, reactive mesothelial cells, histiocytes, lymphocytes.
Right parietal pleural biopsy, 1.9 x 1.5 x .5 cm, reactive mesothelial cells, histiocytes, lymphoid aggregares, hemosiderin pigment and organization.
Negative for malignancy.
Path 2 - AP Cytology
R pleural fluid, 1500 cc amber fluid, benign mesothelial cells and histiocytes, negative for malignancy,
Path 3 - AP Cytology
Right Pleural Fluid 400 cc bloody fluid, benign mesothelial cells, histiocytes, lymphocytes, negative for malignancy, negative for CDX2.
Discussion
In this case report of an asymptomatic right-sided pleural effusion in a 48-year-old man with a history of CRLM being treated with HAIPC, the authors surmise that the hepatic artery infusion pump caused the pleural effusion.
There is a significant complication rate associated with HAIPC, including technical or chemotherapy-related adverse events. In regard to HAIPC-related adverse events, the most common toxicities are diarrhea, neutropenia, and neurotoxicity (2), and other local toxicities are related to hepatobiliary and upper gastrointestinal tract effects (gastritis/duodenitis/PUD) (3). Documented hepatic arterial infusion pump catheter complications occur in approximately 22% and include: arterial thrombosis (6%), catheter occlusion or dislodgement (6%), extrahepatic perfusion or incomplete perfusion (3%), pump site infection or hematoma (3%), and biliary sclerosis (5%) (2, 3).
There is no documentation in the literature involving the association of chronic pleural effusion with HAIPC therapy in patients with metastatic CRC. This paper serves to document this association, as the patient developed the transudative right pleural effusion after having his HAI pump placed and chemotherapy began, and pleural biopsy demonstrated markedly reactive mesothelial cells with chronic and minimal acute inflammation. Of note, this was a diagnosis of exclusion.
The physiology believed to cause the right-sided pleural effusion in this context is sympathetic hepatic inflammation due to local regional chemotherapy. This is supported by the fact that chemically induced hepatitis is common with HAI-FUDR ranging from 30-70% and manifests as serum biochemical abnormalities (3). Although the patient did have normal biliary labs around the time of his surgery, a subclinical hepatitis cannot be excluded, as the liver tissue receiving the toxic therapy likely has minimal function to manifest a hepatitis in the laboratory evaluation. This is reinforced by the fact that normal LFTs do not always mean that the liver is normal, as LFTs can be normal in patients with chronic liver disease, as evidenced by at least 1/3 of patients with chronic hepatitis having persistently normal serum ALT levels despite the presence of inflammation on liver biopsy (4).
In the context of this case report, there is a question of insufficiency of the liver parenchyma owing to tumor burden in the liver as well as tumor necrosis; this would mask an inflammatory response of elevations in LFTs. This local regional toxicity of the diseased liver, which spares the healthy liver remnant, is reinforced by the high hepatic uptake of the chemotherapy administered via the hepatic artery, in which systemic toxicity is minimal (1, 2); this is owed to the fact that liver metastases are perfused almost exclusively by the hepatic artery and normal liver tissue receives its blood supply from mainly portal circulation (1). Furthermore, chemotherapeutic agents used in HAIPC such as FUDR, as in this patient, has a high first pass extraction and short plasma life, therefore systemic toxicity is very low (3).
Interestingly, the most limiting hepatic toxicity related to HAIPC is biliary sclerosis (6), which manifests by elevations in serum alkaline phosphatase, AST/ALT, and bilirubin (2, 5), and occurs in approximately 1-26% of patients (3). One phase II study of adjuvant HAI and oxaliplatin-based systemic therapy with or without bevacizumab was associated with increased biliary toxicity (2). Another study of 475 patients who received HAIPC from 1991-2008 noted a 5.5% incidence of biliary sclerosis. More recently it has been reported that the use of systemic bevacizumab in conjunction with HAI –FUDR significantly increases the risk of biliary sclerosis (3).
One question concerns whether the subclinical hepatitis in this case report is a marker of impending biliary cirrhosis (PBC). PBC is a chronic inflammatory cholestatic disease resulting from genetic and environmental triggers that results in sclerosis of small intrahepatic ducts causing elevations in A, AST, and ALT that are in proportion to the severity of the disease (5). However, in stage 1 PBC there is minimal portal inflammation without florid bile duct lesions (5), therefore it could be argued that in early stages of PBC, there may not be biochemical markers to suggest its presence. Although the patient had normal levels of serum AP, AST/ALT, and bilirubin, he did demonstrate evidence of hepatic inflammation as evidenced by his sympathetic right pleural effusion, suggesting that the patient could be in the early stages of primary biliary sclerosis and may warrant a liver biopsy for definitive diagnosis.
Furthermore, differentiating between a benign and malignant pleural effusion in a cancer patient is essential in a patient with CRLMS being treated with a HAIPC. This is owed to the fact that HAIPC chemotherapy is not recommended in the setting of extrahepatic disease outside of the context of a clinical trial (1). This paper sheds light on being aware of this potential side effect of HAIPC’s in this patient population and not perceiving it as progression of their malignancy.
Conclusion
Recurrent pleural effusions in a patient with HAIPC for CRLM can be a side effect of the regionally directed HAIPC (rather than progression of the malignancy) and may warrant a PleurX catheter placement if the patient is to have continued HAIPC infusions. Subclinical hepatitis, as evidenced by sympathetic pleural effusions, should alert the clinician to the possible development of primary biliary sclerosis in this patient population and may warrant a liver biopsy for diagnosis.
References
- Karanicolas PJ, Metrakos P, Chan K, Asmis T, Chen E, Kingham TP, et al. Hepatic arterial infusion pump chemotherapy in the management of colorectal liver metastases: expert consensus statement. Curr Oncol. 2014;21(1):e129-136.
- Ko YJ, Karanicolas PJ. Hepatic arterial infusion pump chemotherapy for colorectal liver metastases: an old technology in a new era. Curr Oncol. 2014;21(1):e116-121.
- Doussot A, Kemeny NE, D'Angelica MI. Hepatic arterial infusional chemotherapy in the management of colorectal cancer liver metastases. Hepat Oncol. 2015;2(3):275-290.
- Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59(8):2223-2230.
- Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ, et al. Primary biliary cirrhosis. Hepatology. 2009;50(1):291-308.
- Ito K, Ito H, Kemeny NE, Gonen M, Allen PJ, Paty PB, et al. Biliary sclerosis after hepatic arterial infusion pump chemotherapy for patients with colorectal cancer liver metastasis: incidence, clinical features, and risk factors. Ann Surg Oncol. 2012;19(5):1609-1617.
Disclaimer
The information and views presented on CTSNet.org represent the views of the authors and contributors of the material and not of CTSNet. Please review our full disclaimer page here.




