ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
COVID-19 in Cardiac Surgery: A Proposed Prioritization Framework
Karavas A, Downing S. COVID-19 in Cardiac Surgery: A Proposed Prioritization Framework. May 2020. doi:10.25373/ctsnet.12245351
Introduction
As the SARS-CoV-2 pandemic has been reaching unprecedented highs across the US, hospitals have become overwhelmed with patients treated for COVID-19. Hospital resources (beds, equipment, and personnel) have been allocated to control this pandemic and resources for cardiac surgical patients are now limited. Regulatory authorities at regional and national levels have requested that elective cases be cancelled until further notice. The Society of Thoracic Surgeons has added a temporary code for COVID-19 patients to prospectively assess the impact of the disease to cardiac surgery patients and they have excluded these patients from surgeon and program reporting, presumably because of the expected increased morbidity and mortality (1).
The peak of this pandemic has not been reached in the United States, and it is difficult to foresee when hospital operations will resume in a regular manner. Surgeons and patients are not accustomed to waiting long periods of time for their surgery compared to other countries. The median waiting time for a coronary artery bypass grafting (CABG) patient is six days, while in other countries, such as Canada, the UK, and Germany, median waiting time may be as high as 206 days (2). We are now called to adapt in an expeditious manner and prioritize patients, both in urgent as well as elective settings, based on this new reality of very limited resources. The American College of Surgeons has provided some guidance in triaging elective patients in various specialties, but has not addressed cardiac surgery specifically (3).
In the first weeks of imposed measures, the authors’ cardiac surgery department initiated discussions in a multidisciplinary approach on how to alter management of elective and urgent cases. In this report, the authors present data that helps identify the added risks associated with the COVID-19 disease, but also the risk imposed to patients waiting longer for cardiac surgery than they would otherwise have. A prioritization framework based on already published risks scores and guidelines for appropriate use are described as an added tool to supplement thorough patient assessment and sound clinical judgment.
Background
Cardiac disease and coronavirus:
Coronavirus, SARS-CoV-2, is a virus with some similarities to the coronaviruses responsible for previous recent outbreaks: Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). SARS-CoV-2 affects ACE-2 receptors that are present in the heart and the lungs. As many as 28% of COVID-19 hospitalized patient develop signs of acute cardiac injury, reflected in elevation of troponin (4-6). The spectrum of cardiac presentation includes arrhythmias (17%), acute cardiac injury (7% to 28%), acute coronary syndrome without coronary obstruction or stress cardiomyopathy, acute fulminant myocarditis, and cardiac arrest (4-9). First reports indicate that COVID-19 patients with cardiovascular disease have a more severe clinical presentation, are more likely to require ICU admission, and have higher mortality (5-7, 10). Acute coronary syndrome in COVID-19 patients has poor outcome (10). Cardiovascular causes contribute to 40% of the overall mortality in COVID-19 patients (7). More interestingly, COVID-19 patients with pre-existing cardiovascular disease have a reported mortality of 11%, and, if these patients present with acute myocardial injury (reflected in troponin elevation), their mortality increases to 69% (4). As such, cardiac surgery patients, who make up a subset of those, will also be expected to have high mortality.
COVID-19 symptomatic patients:
While there have been no reports on COVID-19 patients undergoing cardiac surgery, one may extrapolate from data on cardiovascular patients with COVID-19 that expectations for mortality, especially in the urgent setting, such as acute coronary syndrome, will be high. A report detailing the MERS outbreak in ICUs included three postoperative cardiac surgery patients, all of whom died (11). These patients had undergone, respectively, CABG, aortic valve replacement (AVR), and pericardiectomy.
Based on this information, the authors are very skeptical about offering cardiac surgery in COVID-19 symptomatic patients as their prognosis is expected to be very poor.
SARS-CoV-2 exposed patients:
SARS-CoV-2 exposed patients are considered those who have symptoms or have been exposed to COVID-19 positive individuals, with or without a confirmed negative test for SARS-CoV-2. These patients pose a great dilemma. The authors anticipate that the perioperative risk in these patients will be increased. Viral infections, such as the flu, have demonstrated increased perioperative morbidity (12). During the MERS epidemic, six asymptomatic patients underwent cardiac surgery, all tested positive for coronavirus, and five of them died (13).
For elective patients, their SARS-CoV-2 status should be confirmed. In urgent patients, it is reasonable to wait for the SARS-CoV-2 testing if the clinical situation allows. The authors also recommend trending cardiac biomarkers (troponin and B-type natriuretic peptide-BNP), as time allows, because continuous rise of those infers poor prognosis (4). These factors, along with temporizing alternatives (percutaneous interventions, medical management) will weigh in the decision for surgical intervention in these patients, disclosing to them and their families that the risk is higher than in normal circumstances. One should be vigilant that clinical and radiographic evaluation should be part of the diagnosis of COVID-19 and not rely solely on a single laboratory test result as the reported false negative rate is significant (14, 15).
SARS-CoV-2 negative patients:
Cardiac surgery patients require a significant amount of resources, including ventilator, intensive care space, and highly trained personnel. Thoughtful resource utilization is important, and the decision tree suggested below is an attempt to account for this. In addition, one needs to assess the urgency of operating in context with potential risk of inoculating SARS-CoV-2 during the admission, which would potentially alter a patient’s outcome. This triage will be a dynamic process accounting for resources, symptom control, and baseline risk. The authors strive for a multidisciplinary approach in deciding acuity in these patients.
Specific Scenarios
Elective patients:
Outpatients awaiting or being prepared for elective cases have already been postponed as their clinical situation allows. The authors have maintained an ongoing open communication in order to stay informed of their clinical status and of any changes that would mandate proceeding with their surgery in a more urgent manner. Telemedicine or even simple phone calls by the surgeons and physician extenders have been implemented early on and have been accepted well by both the medical community and the patients.
Several European countries, Canada, and New Zealand have reported previously on risk stratification and usage of risk models to establish a “maximum waiting period,” or help re-allocate resources towards cardiac surgery or other treatment modalities. The majority of the risk scores involve CABG and/or AVR. These models are intended to assist clinical judgment in balancing between waiting for cardiac surgery and minimize morbidity and mortality associated with this waiting period (16-20). Angina, left ventricular (LV) dysfunction, heart failure, gender, and time on the waiting list are the most common independent predictors of morbidity and mortality during the waiting period in most studies (21).
European guidelines on myocardial revascularization have recommended a simplified approach of a two-week maximum waiting period in patients with CCS-III symptoms or LV dysfunction or complex anatomy (left main or three-vessel CAD with proximal LAD disease), versus a six-week period for all others to be operated on (22). Based on the projections of duration of this pandemic, the authors expect that many of the patients will not be safe to be operated on during these short periods of time. Canadian and New Zealand risk models have been illustrative in triaging patients awaiting CABG and/or AVR and use those to conform their waiting list or to provide a threshold for offering surgery (18-20, 23).
Ray et al. reported on the risk model utilized in the province of Nova Scotia and Prince Edward Island, Canada (24). This classifies CABG and/or AVR patients in four acuity groups; in-house urgent requiring surgery before discharge (unrelenting cardiac compromise unresponsive to other therapies except cardiac surgery), semiurgent-A (those who perform < 2 METs on Standard Bruce Protocol, those with critical left main disease or critical aortic stenosis, but who are deemed stable enough for discharge), semiurgent-B (those who perform 2–5 METs), and elective (those who perform over 5 METs). Based on this classification, median waiting times were roughly one week, one month, two months, and four months for each acuity group respectively with 0.7% overall mortality on the waiting list and 9% of patients requiring acuity stage upgrade and often hospitalization due to clinical deterioration (24).
The risk model proposed by Rexius et al. from Sweden is simple and provides an important clinical adjunct in assessing who can wait for their surgery and for how long. Validation of this model revealed a 0.8% overall mortality during the waiting period. The severity ranges between high score (six points or higher), intermediate score (3-5 points), and low score (0-2 points), and they indicate the need for surgery within two weeks, 12 weeks, and six months, respectively (17).
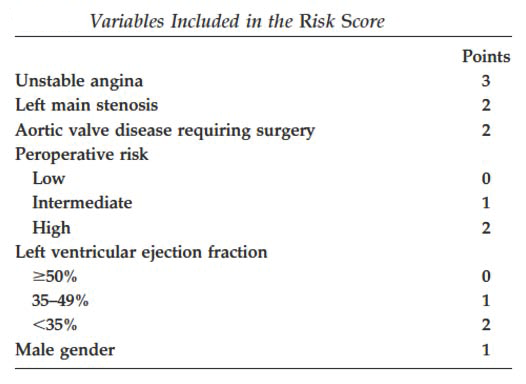
Variables included in Risk Score by Rexius et al. (Reprinted with permission.) (17).
These risk models function as adjuncts to clinical assessment. Being conscientious with resources, the authors evaluate their elective surgical patients and prioritize them within these time frames. Once patients are deemed to need surgery more urgently, their multidisciplinary team assesses clinical urgency, safety, and resource availability to proceed with these cases. This is an evolving mechanism that allows them to create an open communication environment between clinicians, administrators, and patients for proper resource utilization and patient care.
Urgent Cases
Similar to the elective cases, patients considered for cardiac surgery should ideally be tested for SARS-CoV-2, including trending of their troponin and BNP.
- CAD with ST-elevation MI (STEMI) – culprit lesion
Since these patients are usually treated primarily with PCI, CABG is usually reserved for severe cases (multivessel disease, cardiogenic shock). Such patients will be expected to have anatomy that would not allow postponing surgery and a decision will have to be made on their overall condition to withstand CABG rather than timing. Such patients would not be postponed based on exposure status (25). - STEMI – nonculprit lesion during same hospitalization
Following the AHA/ACC guidelines for appropriateness of revascularization, CABG would be considered during index hospitalization (if PCI is not an option) if:
- Patients have spontaneous or easily provoked symptoms, or
- Asymptomatic patients have findings of ischemia on noninvasive testing, or
- Asymptomatic patients have a 50% to 70% lesion and positive FFR (25).
For the latter two groups, clinical judgment (size of myocardium at risk, severity of remaining lesions) and resources will have to be weighed as these patients may benefit from waiting depending on the hospital situation. The Canadian Cardiovascular Society (CCS) and Canadian Wait Time Alliance (CWTA) recommend an upper limit wait-time benchmark of one week for urgent and two weeks for semi-urgent cases (26).
- Unstable angina/Non-ST-elevation MI (NSTEMI)
Patients should be offered CABG if:
a. There is evidence of cardiogenic shock, or
b. Patients are stabilized, but have high risk features (e.g. TIMI Score 3-4) (25).
CCS and CWTA place a two-week upper limit for urgent and semi-urgent cases, and a six-week limit for nonurgent patients with NSTEMI (26).
These patients may be further stratified in low to high risk. High-risk patients (TIMI score 5-7, persistent or recurrent chest pain, moderate or high troponin rise, dynamic EKG changes with chest pain, CHF, hypotension, arrhythmias with chest pain) should be operated on within 24-48 hours. Moderate risk patients (TIMI risk score 3-4, NSTEMI with small troponin leak, worst T-wave inversion or flattening, LV dysfunction (<40%) or previous documented CAD, MI or CABG, or PCI) should be operated on within 1-2 weeks. Lastly, low risk patients (TIMI risk score 1-2, age <65 years, no or minimal troponin, no further chest pain and inducible ischemia < 7METs workload) may be operated on within six weeks (26). These criteria do not recommend CABG over PCI, but provide guidance on those referred to cardiac surgery for consideration for CABG, assuming that PCI was not recommended as the primary treatment choice. In cases where mode of revascularization is in question, a heart team approach will be helpful in guiding decision making.
- Valve disease
Similar to the CABG risk models, some of these models have included patients with need for AVR with or without concomitant CABG. As valve patients appear to have higher mortality while awaiting surgery, more caution in regard to clinical deterioration should be paid (20).
Patients with frank heart failure or symptoms such as syncope or angina with mild exertion should be considered for urgent AVR (by surgical or percutaneous approach) (27). Again, it is reasonable to evaluate their SARS-CoV-2 status prior to cardiac surgical intervention. Whether aortic stenosis patients are to be considered primarily for transcatheter over surgical aortic valve replacement will be decided using a heart team approach and consideration of current resources. Anecdotal reports indicate that the current tendency is towards transcatheter valve due to the lower resource utilization and shorter hospital stay. Based on the CWTA and CCS, the current wait-time benchmark for nonemergent valve surgery is two weeks in patients with critical symptomatic aortic stenosis and six weeks for all other nonurgent-outpatient valve patients (26). Prioritization is given to those with worsening NYHA class, are unresponsive to medical treatment, or have frank failure that may require hospital ad mission and surgical management.
- Other cardiac surgical pathology
Patients with endocarditis and aortic dissection are less likely to deviate from current guidelines since the primary disease is more life-threatening and requires prompt intervention. Again, knowing SARS-CoV-2 is important, especially in those in whom mortality is already expected to be high.
ECMO and Coronavirus
Early reports from China confirm low usage of ECMO, which may be attributed to low need (by indication or predicted success), late institution, inadequate experience in that specific setting, or overutilization of the already scarce resources (6). ELSO has been proactive and has already initiated an effort to create a real-time database to assist with management of such patients (28).
First reports indicate that initially lung compliance is such that patients are able to maintain oxygenation with standard ventilation methods including proning. Prior epidemics such as MERS in Saudi Arabia have described encouraging results (29). While it is not the purpose of this report to go into depth on ECMO management, the following points are useful for the practicing cardiac surgeon, and most have been well summarized in the ELSO guidance (28).
Inexperienced centers should avoid initiating ECMO programs during this pandemic. ECMO should be considered in patients with high risk of mortality, preferentially early in the disease process, younger patients, patients with less comorbidities, and also health care workers. Be aware of the possibility of cardiac decompensation following respiratory failure, as direct cardiac involvement has been described and should be considered in the management of these patients. While indications are similar to those with severe ARDS due to other pathogens, allocation of resources towards ECMO and especially CPR-ECMO should be based on local decisions and available resources (28).
Conclusion
These are challenging times at every level and in every specialty that affect medical professionals and patients equally. Cardiac surgeons utilize a significant amount of resources. During this pandemic, the majority of cardiac surgical procedures have been postponed. This creates a significant backlog of patients waiting for surgery that will persist longer than the pandemic. Operating on younger, healthier, lower-risk patients, just to decrease the backlog, utilizes much needed resources, exposes them to a considerable risk of SARS-CoV-2 inoculation, and should be avoided. This report is designed to provide a critical review of factors that affect decision and timing for surgery. It is meant to be a review of current literature and provide a framework to build additional information as the experience with COVID-19 grows. Still, the three major factors in balancing risk of operating, waiting, and resource utilization is the use of sound clinical judgment, risk stratification with a model, and a multidisciplinary approach with colleagues from other disciplines, including those overseeing hospital resources.
References
- Society of Thoracic-Surgeons. STS Responds to the COVID-19 Crisis — March 31, 2020 2020.
https://www.sts.org/publications/videos/sts-responds-covid-19-crisis-march-31-2020 - Ott E, Mazer CD, Tudor IC, Shore-Lesserson L, Snyder-Ramos SA, Finegan BA, et al. Coronary artery bypass graft surgery—care globalization: The impact of national care on fatal and nonfatal outcome. The Journal of Thoracic and Cardiovascular Surgery 2007;133:1242–51. doi:10.1016/j.jtcvs.2006.12.031.
- COVID19: Elective Case Triage Guidelines for Surgical Care 2020
https://www.facs.org/covid-19/clinical-guidance/elective-case - Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020. doi:10.1001/jamacardio.2020.1017.
- Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020. doi:10.1001/jamacardio.2020.0950.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. Jama 2020;323:1061. doi:10.1001/jama.2020.1585.
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality dueto COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine 2020:1–3. doi:10.1007/s00134-020-05991-x.
- Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. European Heart Journal 2020. doi:10.1093/eurheartj/ehaa190.
- Zeng JH, Liu Y-X, Yuan J, Wang F-X, Wu W-B, Li J-X, et al. First case of COVID-19 infection with fulminant myocarditis complication: case report and insights. Preprints 2020:1–15. doi:10.20944/preprints202003.0180.v1.
- Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nature Reviews Cardiology 2020:1–2. doi:10.1038/s41569-020-0360-5.
- Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 2014;160:389–97. doi:10.7326/M13-2486.
- Groeneveld GH, van Paassen J, van Dissel JT, Arbous MS. Influenza Season and ARDS after Cardiac Surgery. N Engl J Med 2018;378:772–3. doi:10.1056/NEJMc1712727.
- Nazer RI. Outbreak of Middle East Respiratory Syndrome-Coronavirus Causes High Fatality After Cardiac Operations. The Annals of Thoracic Surgery 2017;104:e127–9. doi:10.1016/j.athoracsur.2017.02.072.
- Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020:200642. doi:10.1148/radiol.2020200642.
- He F, Deng Y, Li W. Coronavirus Disease 2019 (COVID‐19): What we know? J Med Virol 2020:jmv.25766. doi:10.1002/jmv.25766.
- Hadorn DC, Holmes AC. The New Zealand priority criteria project. Part 2: Coronary artery bypass graft surgery. Bmj 1997;314:135–8. doi:10.1136/bmj.314.7074.135.
- Rexius H, Brandrup-Wognsen G, Nilsson J, Odén A, Jeppsson A. A Simple Score to Assess Mortality Risk in Patients Waiting for Coronary Artery Bypass Grafting. The Annals of Thoracic Surgery 2006;81:577–82. doi:10.1016/j.athoracsur.2005.08.032.
- Seddon M, Broad J, Crengle S, Bramley D, Jackson R, White H. Coronary artery bypass graft surgery in New Zealand's Auckland region: a comparison between the clinical priority assessment criteria score and the actual clinical priority assigned. N Z Med J 2006;119:U1881.
- Naylor CD, Basinski A, Baigrie RS, Goldman BS, Lomas J. Placing patients in the queue for coronary revascularization: evidence for practice variations from an expert panel process. Am J Public Health 1990;80:1246–52. doi:10.2105/ajph.80.10.1246.
- Morgan CD, Sykora K, Naylor CD. Analysis of deaths while waiting for cardiac surgery among 29,293 consecutive patients in Ontario, Canada. The Steering Committee of the Cardiac Care Network of Ontario. Heart 1998;79:345–9.
- Head SJ, da Costa BR, Beumer B, Stefanini GG, Alfonso F, Clemmensen PM, et al. Adverse events while awaiting myocardial revascularization: a systematic review and meta-analysis. European Journal of Cardio-Thoracic Surgery 2017;52:206–17. doi:10.1093/eurheartj/eht059.
- Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. European Heart Journal 2018;40:87–165. doi:10.1016/j.ahj.2013.07.035.
- Seddon ME, French JK, Amos DJ, Ramanathan K, McLaughlin SC, White HD. Waiting times and prioritization for coronary artery bypass surgery in New Zealand. Heart 1999;81:586–92. doi:10.1136/hrt.81.6.586.
- Ray AA, Buth KJ, Sullivan JA, Johnstone DE, Hirsch GM. Waiting for cardiac surgery: results of a risk-stratified queuing process. Circulation 2001;104:I92–8.
- Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2016 Appropriate Use Criteria for Coronary Revascularization in Patients With Acute Coronary Syndromes: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society of Thoracic Surgeons. Journal of the American College of Cardiology 2017;69:570–91. doi:10.1016/j.jacc.2016.10.034.
- CCS-Access-to-Care-Working-Group. Wait-time benchmarks for cardiovascular services and procedures 2006:1–21.
https://www.ccs.ca/images/Documents_2015/Wait_Times_Benchmarks.pdf - Otto C, Cooper S. Medical management of symptomatic aortic stenosis. UpToDate 2019:1–15.
- ELSO. ELSO ECMO guidance 2020:1–4.
https://www.elso.org/Portals/0/Files/pdf/ECMO%20for%20COVID%2019%20Guidance%20Document.Final%2003.24.2020.pdf - Alshahrani MS, Sindi A, Alshamsi F, Al-Omari A, Tahan El M, Alahmadi B, et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Annals of Intensive Care 2018:1–10. doi:10.1186/s13613-017-0350-x.
Disclaimer
The information and views presented on CTSNet.org represent the views of the authors and contributors of the material and not of CTSNet. Please review our full disclaimer page here.




