ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
COVID-19: Chest Drains With Air Leak – The Silent ‘Super Spreader’?
Bilkhu R, Viviano A, Saftic I, Billè A. COVID-19: Chest Drains With Air Leak – The Silent ‘Super Spreader’?. April 2020. doi:10.25373/ctsnet.12089130
To date, one million confirmed cases of SARS-CoV-2 virus have been reported worldwide with a death toll of over 50,000 (1). Particular concern has been raised regarding the exposure of healthcare professionals. Early reports from the Wuhan province in China described up to 29% infection rates among healthcare professionals before the use of personal protection equipment (PPE) was fully established (2). Several measures are being established with regard the correct use of PPE and reduction in aerosol generating procedures. However, to the authors’ knowledge, no specific guidance is available regarding the potential risk of aerosolization of SARS-Cov-2 virus via chest drains in patients with active air leak.
Viral Spread and Air Leak
The SARS-CoV-2 virus, which leads to COVID-19, has been demonstrated to remain viable in aerosol form and is transmitted by droplets (3). Despite the current coronavirus pandemic, we are still faced with patients requiring chest tube drainage for pneumothorax on cardiothoracic and respiratory wards, as well as in critical care units. Whilst drains may be inserted with lower risk of viral spread for simple pleural effusions, the authors fear there may be a high risk of aerosolization in cases of pneumothorax with active air leak, whether that be primary, secondary, or indeed iatrogenic in mechanically ventilated patients requiring high PEEP ventilation, such as in patients with COVID-19.
Citing a recent example of a postoperative thoracic surgical patient in the authors’ unit who had a prolonged air leak and who later was found to be positive for SARS-CoV-2, they have considered the implications of aerosolization from the chest drain and in particular the chest drain bottle. This may represent an under-recognised means of viral spread, which may put patients and health care professionals at risk of infection.
Chest Drains and Risk of Aerosolization
Traditional under water seal chest drain bottles have a port which allows attachment to low pressure wall suction. Most modern drain systems also have a safety valve which opens to air should the suction be accidentally turned off in the presence of an air leak, to avoid creating a closed system effect which could lead to a tension pneumothorax. If the drain bottle is not attached to suction, then the port is open to the atmosphere.
When air leaks into a chest drain bottle, it causes the fluid inside to bubble. Given the aerosolization that is likely to occur inside the drain bottle, which then escapes through the suction port or safety valve, this may be a potentially important mode of viral transmission. Alternatives to a traditional chest drain bottle include a number of different digital chest drainage systems. Whilst these do not have a port open to room air, they are not closed systems and the air escapes from the system into the air without any specific viral filter.
A number of patients on the authors’ unit’s thoracic ward have since tested positive for COVID-19. Whilst the patient with the air leak may not have been the source of infection, they feel this should be considered. In their patient, a digital chest drainage system was being used.
In light of this, and until further robust evidence regarding the volume of aerosolization from a chest drain bottle emerges, the authors would recommend the use of closed drainage systems, i.e. connecting the standard drain bottle to wall suction to avoid the spread of viral load via aerosolization. However, in order to obtain this, the safety valve will have to be occluded with potential risk of increasing intrathoracic pressure and cause tension, should the suction system be switched off whilst still connected to the bottle. Furthermore, keeping the bottle attached to wall suction will significantly limit the mobilization of patients, which is a significant risk factor for postoperative complications in the surgical patient.
A Bespoke Chest Drain System
In order to overcome this, a possible consideration would be to attach an antimicrobial filter, such as those used in ventilator circuits, to the chest drain suction port leaving the drain off suction and occluding the safety valve. Connecting the filter directly to the chest drain should be discouraged, as fluid and moisture directly from the chest cavity are likely to interfere with the functioning of the filter.
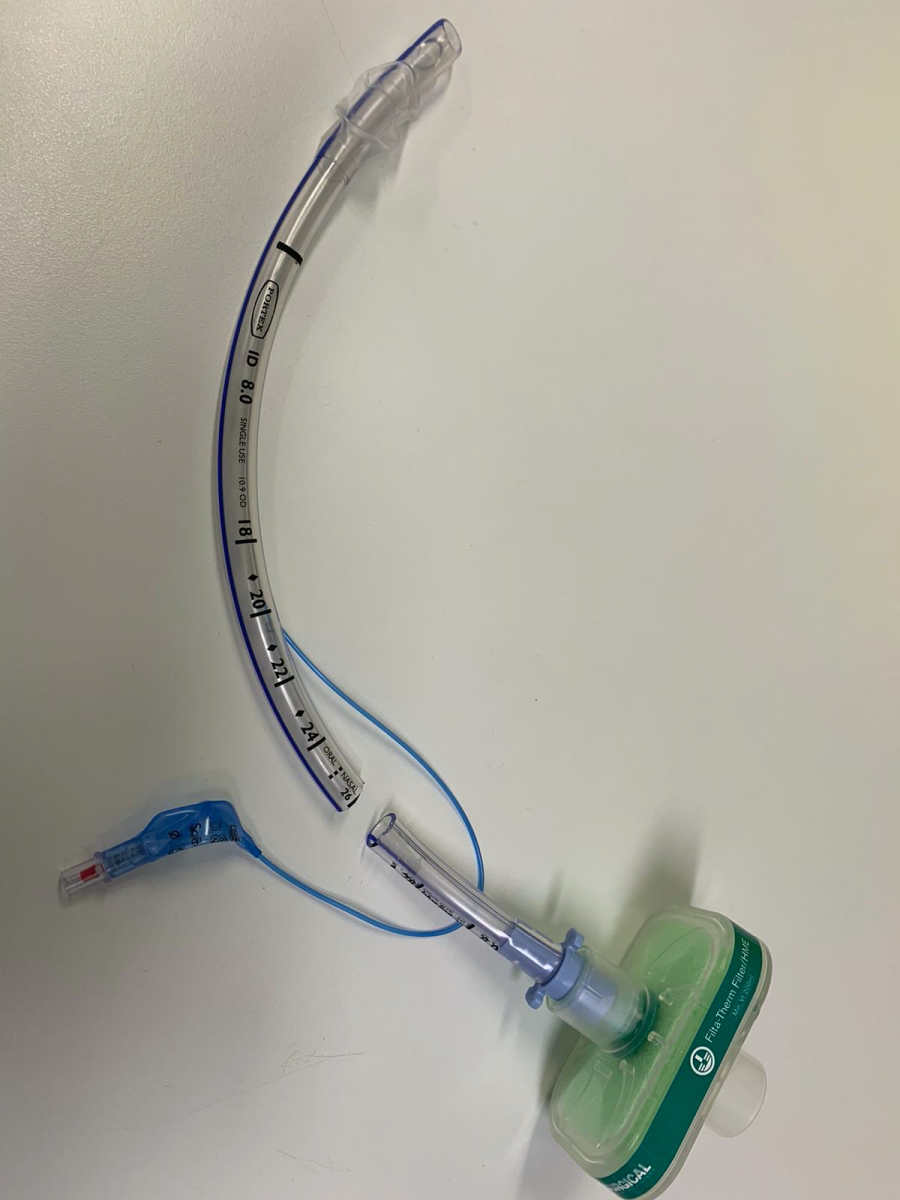
Therefore, the authors designed a bespoke drainage system using the Filta-Guard™ ventilator filter from Intersurgical Ltd© 2020 and a segment of endotracheal tube to use in their unit (Figures 1 and 2). The filter guarantees a filtration efficiency of >99.999% as tested on Hepatitis C and Mycobacterium tuberculosis in addition to standard test micro-organisms (4). The SARS-Cov-2 diameter varies from 60 to 140 nm, and therefore is larger than Hepatitis C virus, which has an average diameter of about 55 nm. The authors postulated that given the larger size compared to Hep C virus, this filter should be effective in preventing flow of SARS-Cov-2 across the filter; however to their knowledge, this has not been clinically tested. Regarding the possible resistance to the system added by the filter and related risk of building up pressure in the chest cavity, they believe this should be marginal. Published data suggest the above filter would generate a resistance against the passage of 30L/min of 1.0cm H2O and 2.3cm H2O at 60L/min (4).
Conclusions
The efficacy of this chest drain modification clearly needs to be further investigated. However, given the current pandemic, any method of reducing viral spread should be considered.
Acknowledgements:
The authors would like to acknowledge Mr Panagiotis Theodoropoulos and Mr Duncan Steele, Specialist Registrars in Thoracic Surgery at Hammersmith Hospital, London.
References
- Johns Hopkins University & Medicine. COVID-19 Map. https://coronavirus.jhu.edu/map.html. Published 2020. Accessed April 2, 2020.
- Chen W, Huang Y. To protect healthcare workers better, to save more lives. Anesth Analg. 2020:1-15. doi:10.1213/ANE.0000000000004834
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020. doi:10.1056/NEJMc2004973
- Systems ICR. Filta-GuardTM range - high efficiency. https://www.intersurgical.com/products/airway-management/filtaguard-rang.... Published 2020. Accessed April 2, 2020.
Disclaimer
The information and views presented on CTSNet.org represent the views of the authors and contributors of the material and not of CTSNet. Please review our full disclaimer page here.






Comments