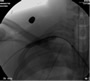
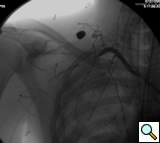
Traumatic subclavian artery transection is a potentially lethal injury following either blunt or penetrating injury to the chest. In one series [1], it accounted for 9 of 39 (23%) cases of overall thoracic vascular injuries and 9 of 411 (2.1%) of overall vascular injuries and has the potential to result in hemothorax or exsanguinations with a quoted mortality ranging from 5 to 30% [2]. Traditional open surgical repair has been the mainstay of therapy [2, 3] however, covered stent graft repair may represent a newer less invasive alternative
Diagnosis of traumatic subclavian artery transection is made by history with correlation of clinical exam with a pulse deficit of the ipsilateral upper extremity. Hard signs include severe external hemorrhage, expansile hematoma, palpable thrill, continuous murmur and any of the classic findings of acute arterial ischemia. Soft signs include nonpulsatile and nonexpanding hematoma, ipsilateral neurologic deficit or history of hemorrhage or proximity of a major vascular structure. Axial and multiplanar images by contrast enhanced computed tomography scan may assist in formulating an open surgical strategy versus an endovascular intervention and assist in appropriate sizing of the native vessels for endovascular stent graft repair. Aortic arch anomalies such as a bovine arch, anomalous vertebral artery, arch with severe atheromatous changes or an aberrant right subclavian artery may preclude a transfemoral approach and instead dictate access via the ipsilateral brachial artery.
OPERATIVE STEPS
The patient was an 18-year-old male that presented with a gun shot wound to the right upper extremity. The patient will undergo appropriate trauma resuscitation with fluids and medications per institutional trauma and ATLS guidelines [4]. Patients may or may not be intubated with general endotracheal anesthesia. In our institution, the procedure is performed in the operating room in case open surgical conversion is necessary. We utilize the OEC 9900 Elite(GE Medical Systems. Milwaukee, WI) C-arm intraoperatively.
The patient is positioned supine with the ipsilateral upper extremity prepped out at a 90º angle and with both groins and chest prepped in as well. The right femoral artery is percutaneously engaged in a standard Seldinger technique with a standard short 5F sheath. We utilize an 0.035 inch hydrophilic angled Glidewire® (Terumo Medical Corporation, Somerset, NJ), which is brought up into the ascending aorta and followed with a JR4(C.R. Bard Inc. Murray Hill, NJ) catheter. The C-arm is positioned in a 25º LAO view to “open the arch” and the aortogram is commenced delineating the arch and arch vessel anatomy. Gentle traction and pullback on the catheter allows us to engage the target arch vessel, selectively advancing the Glidewire into the appropriate vessel. Next, once the wire is placed far distally the catheter is gently advanced into the appropriate vessel where selective angiograms are performed (Figure 1). Inability to traverse the transected subclavian artery may mandate a retrograde transbrachial approach.
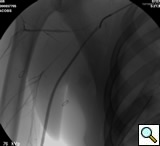 |
| Figure 2: Post deployment of angled glide wire and JR4 catheter past the transected subclavian artery revealing patent vessel distally. |
After traversing the vessel with a Glidewire and visualizing the wire conforming to normal arterial profile down into the distal extremity, it is necessary to change out the sheath for a long 8F sheath. It is necessary to advance the sheath into the proximal subclavian artery. To increase support we change out the guide wire for a stiffer Amplatz® (Boston Scientific Corp. Natick, MA) Guidewire extending down into the arm. Next, we perform selective views of the
transected artery visualizing the pseudoaneurysm (Figure 2). A Viabahn covered stent (W.L. Gore & Associates, Flagstaff, AZ) is chosen for the appropriate size of the native vessel being careful not to oversize the stent graft. The stent graft is advanced across the lesion. Selective angiograms through the sheath may be performed to choose correct placement and appropriate landing zones. The stent graft is deployed under fluoroscopic visualization. Next, we utilize an appropriate sized 10 mm low pressure balloon to “iron out the edges” of the stent and bring it up to profile of the artery (Figure 3). Next, completion angiograms are performed to rule out endoleak and complete exclusion of the pseudoaneurysm, as well as to confirm distal runoff to the forearm and palmar branches of the extremity (Figure 4).
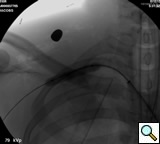 |
| Figure 4: Post-deployment of the stent with 10 mm balloon brought up to vessel profile. |
Sheath and wire are pulled back into the iliac artery under fluoroscopic visualization. After an appropriate “sheath shot” angiogram to delineate correct placement of the arterial puncture, the sheath is changed out to an 8F Angioseal ® (St. Jude Medical Inc. St. Paul, MN) closure device.
Discussion
Over the last decade, catheter-based and endovascular techniques have been utilized with increasing frequency in the management of blunt and penetrating trauma. There is a large body of evidence involving traditional open procedures [2, 3] with overall cervicothoracic arterial injuries secondary to trauma with an overall mortality of 30%. Demetraides group found that subclavian venous injuries were just as lethal [3]. High velocity penetrating wounds were associated with a higher mortality as well when compared to low velocity penetrating wounds [5].
Stent graft therapy was originally employed to treat the subclavian artery for trauma [6]. The use of covered stents in trauma of the subclavian and axillary arteries is not new and with use of these conduits from large volume trauma centers is associated with a shorter operative time and less blood loss [7] while achieving a 100% technical success rate [8] and 100% primary patency over a mean of 22 months with no device fractures or migration. Danetz’ group [9] estimated that close to 50% of the traumatic axillosubclavian injuries could be treated in this fashion, however this was a somewhat dated retrospective review.
Aims of vascular stenting are to occlude the walls of vital arteries without compromising flow, exclude pseudoaneurysms or fistulas and prevent backflow of embolic materials into vital arteries after branch embolization. The advantages of an endovascular approach is from a distant non-injured site; avoids the morbidity from surgical access and difficult dissection and repair in injured tissue. It may be most beneficial in the critically ill where anesthesia or vascular reconstruction may be hazardous. Endovascular repair obviates the need for thoracotomy, clavicular resection and or sternotomy. The advantages of endovascular therapy to treat subclavian injuries avoids major operative dissection in the traumatized area eliminating the risk of significant hemorrhage from arteriovenous fistulae and minimizing the possibility of injury to important surrounding structures such as the subclavian vein and brachial plexus. Seventy-three percent of the Stanford series [10] had a serious illness or a major associated trauma. These may be difficult to identify secondary to hematoma within the surrounding tissues and often with suboptimal vessel exposure. In the Montefiore group study of 7 patients were either treated via open access or percutaneously and they assert there is associated decreased blood loss, reduced requirements for anesthesia and a clearly limited need for dissection in a traumatized field [11].
Marin in his series treated 8 patients with traumatic arterial lesions (2 arteriovenous fistulae and 6 pseudoaneurysms) with endoluminal stent grafts with a technical success of 95% [12]. Most of the studies are all of limited numbers. Hilfiker, et al., [10] reported on 9 patients with aneurysms or fistulas and all were treated successfully with stent grafts and had an associated 89% and 100% primary and secondary patency respectively. Interestingly, 6 of the patients in this series were traumatic secondary to iatrogenic causes and the authors utilized stent diameters of 7 mm to 12 mm (mean 10 mm) and brachial access in 33% of cases. Some series showed a majority of the access via the brachial artery. Brachial access albeit a more direct access presents some clinical problems such as a higher incidence of hematoma or pseudoaneurysm and resultant neuropathy. Open cutdown may decrease the risk.
Presence of subclavian branches are important considerations as well. One must keep proximity and possible vertebral artery sacrifice in mind when the vertebral artery lies near a subclavian lesion and may be potentially covered. Also, the presence of or the future use of an internal mammary artery graft may also be a strong consideration as well.
There are numerous documented reports of stent graft therapy for arteriovenous malformations (AVM). Due to resistant arterial feeders into the AVM, skeletonization of AVM by the graft may not be a durable solution secondary to the possibility of endoleak and further embolization may be more difficult.
Beregi’s group looked at treatment of peripheral aneurysms of which 5 of 19 were to the subclavian artery [13]. Overall aneurysm groups including iliacs, subclavian, femoral and popliteal had successful exclusion of the aneurysm in 95% of the cohort proving a clear efficacy for covered stent grafts. Henry in his study looked at the efficacy of covered stents as an “interval bypass” in iliac and femoral-popliteal segments and achieved technical success in 98% of his series [14].
Szeimies, et al., [15] described 2 cases of subclavian artery aneurysm exclusion with 100% technical success, however both patients went on to develop stenoses with one being successfully treated with angioplasty. Patel in his study looked at penetrating injuries to the subclavian artery in 6 patients and had stent grafts deployed to cover 5 pseudoaneurysms and one AVF with 100% technical success with complete exclusion and one patient went on to develop stent fracture at 8 months treated by a second covered stent [16]. Sullivan, et al., [17] in his group of terminal patients with significant medical comorbidities had 100% exclusion of axillary and subclavian aneurysm with covered stents.
Disadvantages of the endovascular approach include graft durability and complications over the lifespan of mostly young patients and durability is unknown over a lifetime of dynamic motion in the cervicothoracic branches. Grafts can migrate and many are associated with a 5-20% endoleak rate in proximal lesions. Long-term effects of covering the subclavian origin and vertebral artery are unknown. There have been no large trials been done validating this technique and there is no real published data regarding the infectious risk of the stentgraft in patients with large entry and exit wounds.
The Gore Viabahn Endoprosthesis® (W.L. Gore & Associates, Flagstaf, AZ) was originally approved in July 2005 and is made of an expanded polytetrafluoroethylene or ePTFE lining to limit in-stent restenosis with a peripheral nitinol self-expanding stent on the exterior. It comes in 2.5 mm, 5mm, 10cm and 15 cm lengths. Primary patency overall of the Viabahn is quoted at 80% at one year and 62% at 5 years but this is in superficial femoral artery lesions for occlusive disease [18]. While the Viabahn® is currently indicated for the superficial femoral artery, there are numerous reports of use in the SVC, IVC, hemodialysis grafts, popliteal artery, carotid, hepatic and subclavian arteries [7, 18-25].
Technical skills, stent technology and delivery systems continue to evolve and are a new exciting and expanding field. Success requires combined skills of surgeons and interventionalists and new systems and protocols will develop as the data matures.
References
- Bongard F, Dubrow T, Klein S. Vascular injuries in the urban battleground: experience at a metropolitan trauma center. Ann Vasc Surg 1990;4:415-18.
- Demetriades D, Chahwan S, Gomez H, et al. Penetrating injuries to the subclavian and axillary vessels. J Am Coll Surg 1999;188:290-95.
- George SM Jr, Croce MA, Fabian TC, et al. Cervicothoracic arterial injuries: recommendations for diagnosis and management. World J Surg 1991;15:134-39.
- ATLS Advanced Trauma Life Support Program for Doctors (Paperback) American College of Surgeons; 7th edition, January 2004.
- Degiannis E, Velmahos G, Krawczykowski D, Levy RD, Souter I, Saadia R. Penetrating injuries of the subclavian vessels. Br J Surg 1994;81:524-26.
- Dotter CT. Transluminally-placed coilspring endarterial tube grafts: long-term patency in canine popliteal artery. Invest Radiol 1969;4:329-32.
- Castelli P, Caronno R, Piffaretti G, Tozzi M. Emergency endovascular repair for traumatic injury of the inferior vena cava. Eur J Cardiothorac Surg 2005;28:906-8.
- Castelli P, Caronno R, Piffaretti G, et al. Endovascular repair of traumatic injuries of the subclavian and axillary arteries. Injury 2005;36:778-82.
- Danetz JS, Cassano AD, Stoner MC, Ivatury RR, Levy MM. Feasibility of endovascular repair in penetrating axillosubclavian injuries: a retrospective review. J Vasc Surg 2005;41:246-54.
- Hilfiker PR, Razavi MK, Kee ST, Sze DY, Semba CP, Dake MD. Stent-graft therapy for subclavian artery aneurysms and fistulas: single-center mid-term results. J Vasc Interv Radiol 2000;11:578-84.
- Marin ML, Veith FJ, Panetta TF, et al. Transluminally placed endovascular stented graft repair for arterial trauma. J Vasc Surg 1994;20:466-72.
- Marin ML, Veith FJ. Clinical application of endovascular grafts in aortoiliac occlusive disease and vascular trauma. Cardiovasc Surg 1995;3:115-20.
- Beregi JP, Prat A, Willoteaux S, Vasseur MA, Boularand V, Desmoucelle F.
Covered stents in the treatment of peripheral arterial aneurysms: procedural results and midterm follow. Cardiovasc Intervent Radiol 1999;22:13-19.
- Fischer M, Schwabe C, Schulte KL Value of the Hemobahn/Viabahn endoprosthesis in the treatment of long chronic lesions of the superficial femoral artery: 6 years of experience. J Endovasc Ther 2006;13:281-90.
- Szeimies U, Kueffer G, Stoeckelhuber B, Steckmeier B. Successful exclusion of subclavian aneurysms with covered nitinol stents. Cardiovasc Intervent Radiol 1998;21:246-49.
- Patel AV, Marin ML, Veith FJ, Kerr A, Sanchez LA. Endovascular graft repair of penetrating subclavian artery injuries. J Endovasc Surg 1996;3:382-88.
- Sullivan TM, Bacharach JM, Perl J, Gray B. Endovascular management of unusual aneurysms of the axillary and subclavian arteries. J Endovasc Surg 1996;3:389-95.
- Rajasinghe HA, Tzilinis A, Keller T, Schafer J, Urrea S. Endovascular exclusion of popliteal artery aneurysms with expanded polytetrafluoroethylene stent-grafts: early results. Vasc Endovascular Surg 2007;40:460-66.
- Azizzadeh A, Pham MT, Estrera AL, Coogan SM, Safi HJ. Endovascular repair of an iatrogenic superior vena caval injury: a case report. J Vasc Surg. 2007;46:569-71.
- Vesely TM. Use of stent grafts to repair hemodialysis graft-related pseudoaneurysms.
- Ahuja V, Tefera G. Successful covered stent-graft exclusion of carotid artery pseudo-aneurysm: two case reports and review of literature. Ann Vasc Surg 2007;21:367-72.
- Gupta K, Dougherty K, Hermman H, Krajcer Z. Endovascular repair of a giant carotid pseudoaneurysm with the use of Viabahn stent graft. Catheter Cardiovasc Interv 2004;62:64-68.
- Singh CS, Giri K, Gupta R, Aladdin M, Sawhney H. Successful management of hepatic artery pseudoaneurysm complicating chronic pancreatitis by stenting. World J Gastroenterol 2006;12:5733-34.
- Kasirajan K, Matteson B, Marek JM, Langsfeld M. Covered stents for true subclavian aneurysms in patients with degenerative connective tissue disorders. J Endovasc Ther 2003;10:647-52.
- Xenos ES, Freeman M, Stevens S, Cassada D, Pacanowski J, Goldman M. Covered stents for injuries of subclavian and axillary arteries. J Vasc Surg 2003;38:451-54.