ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Management of a Left Ventricular Aneurysm in an Adult
By Sorin V. Pusca, MD, Barry C. Esrig, MD, Preet M.S. Randhawa, MD, Dusan Knezevic, MD , and Muhamed Saric, MD
Patient Presentation
A 65-year-old female presented to the emergency department with acute respiratory failure due to pulmonary edema. She had a history of several months of worsening dyspnea on exertion. She was admitted to the intensive care unit, intubated, and received aggressive diuresis and afterload reduction therapy. After several days, she was successfully extubated and cardiac catheterization was performed.
Her past medical history was significant for hypertension, non-insulin dependent diabetes mellitus, and a remote myocardial infarction. She denied use of tobacco or alcohol and denied history of chest pain, orthopnea, and lower extremities edema. Her medications after extubation included Lasix, Aldactone, Coreg, Vasotec, Zocor, Aspirin and Glucovance.
On this regimen, she had no orthopnea at bedrest; her heart rate was 60 beats/min, regular, and the blood pressure was 124/60 mm Hg. She had no jugular venous distension or carotid bruits. She did not use her accessory respiratory muscles, but she had bibasilar rales in the lower quarter of her lung fields. She had a left parasternal systolic ejection murmur grade II/VI without radiation. She had neither hepatosplenomegaly nor pretibial edema. Her peripheral pulses were normal, and she had no focal neurologic deficits.
Her blood urea nitrogen was 29 mg/dl, and serum creatinine was 0.9 mg/dl. The electrocardiogram showed normal sinus rhythm at 69 beats/min and Q waves in leads II, III and aVF. Her pulmonary function test showed a vital capacity of 1.5l (51% predicted), a forced expiratory volume in one second of 1.4l (59% predicted), and a maximal voluntary ventilation of 32.9 l/min (37% predicted). Her chest roentgenogram showed small bilateral pleural effusions and pulmonary vascular congestion.
The cardiac catheterization showed left anterior descending artery (LAD) 95% stenosis, obtuse marginal artery (OM) 80% stenosis and proximal right coronary artery (RCA) 85% stenosis. The left ventricular (LV) ejection fraction was 30% and an apical aneurysm could be clearly identified. The preoperative transthoracic echocardiogram without and with intravenous contrast confirmed a 3.5x 1.5 cm left ventricular aneurysm. The left ventricular (LV) ejection fraction was estimated at 25-30% with a dyskynetic apex, inferior and posterior hypokinesis, grade one LV diastolic dysfunction, and 1+ mitral regurgitation. No intracardiac thrombus was appreciated. The LV end-diastolic diameter was 6.2 cm (normal < 5.2cm), the LV end-systolic diameter was 4.8 cm (normal < 2.3-3.9 cm) and the LV end-systolic volume index (LVESVI) was 70.1 ml/m2. The left atrium measured 4.0 cm in diameter. The aortic, pulmonary, and tricuspid valves were normal and the estimated pulmonary artery systolic pressure was 20 mm Hg. Preoperative non-invasive vascular studies showed no significant carotid stenosis and normal ankle-brachial indices.
In summary, this is a patient in class III NYHA secondary to ischemic cardiomyopathy– currently hospitalized on maximal medical therapy. She has single segment dyskinesia with one-third of the anterior wall dyskinetic/akinetic forming a left ventricular apical aneurysm.
She underwent surgical anterior ventricular restoration (SAVER) and coronary revascularization (left internal mammary artery to left anterior descending artery, and two saphenous vein grafts from the aorta to an obtuse marginal and right coronary artery, respectively).
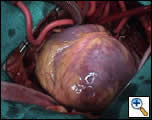
The operation was performed with bi-caval cannulation and an LV vent via the right superior pulmonary vein. Myocardial protection was achieved with antegrade aortic and vein graft blood cardioplegia, as well as retrograde blood cardioplegia. Prior to arresting the heart it was noticed that the apical aneurysm was an akinetic, but not thinned segment of the heart (Figure 1) and as such its margins could not be easily identified on the arrested heart. The aneurysm margins were delineated after completion of distal anastomoses and cross clamp removal, by opening the apex of the beating heart. A pursestring Fontan suture was passed through the base of the aneurysm and the opening closed with a sized Dacron patch; the aneurysmal tissue was imbricated over the repair. The bypass time was 111 min with a cross-clamp time of 44 minutes.
Postoperatively, there were no arrhythmias. The patient was off all inotropes and pressors on postoperative day two and discharged home on post-operative day six. Seven months after surgery she is in NYHA class I and the echocardiogram shows restoration of ventricular geometry.
Comments
The concept of excluding the akinetic portion of the ventricle derives from the fact that when a portion of the ventricular wall is transformed into scar tissue, the loss of function is higher then estimated by the loss of contractile tissue alone. This is due to changes in the ventricular geometry and loss of synchronization of ejection phases that alter fundamentally the muscle mechanics of the ventricle [1]. It was noted that excluding that portion of the ventricle by resection, plication or resection could improve ventricular function. Cooley described a resection and linear closure of aneurysms in 1958; for aneurysms in the proximity of the ventricular septum, he described a septal inclusion technique. Jatene described a technique of septal imbrication in 1985 and Cooley described a technique for septal exclusion in 1988. It was not, however, until Dor in 1985 that the concept of preservation of left ventricular geometry after such resections/exclusions became clear [2]. This began the era of SAVER and made possible excision of myocardial segments that were akinetic but had no scar as in this case.
In relatively small aneurysms, survival is influenced by symptoms at presentation; asymptomatic patients have a five-year survival in 85-89% range while symptomatic patients have a survival in 55-59% range [3]. Medical treatment can extend 50% survival to 6.5 years (ace-inhibitors), 7.5 years (beta-blockers) and 8.5 years (spironolactone) [4] and heart transplantation can extend this further to 9.1 years for advanced class NYHA patients [5].
Overall 5-year survival after the SAVER operation is 69% [4]. The preoperative left ventricular end-systolic volume index (LVESVI) is a critical measurement in planning the SAVER operation. Patients with symptoms of heart failure but LVESVI < 60 ml/m2 should not undergo ventricular remodeling, as ventricular size may become too small. In contrast, patients with preoperative LVESVI > 100 ml/m2 have a poor long-term outcome from bypass alone [6], and when SAVER is indicated, have been shown to have an increased survival. Patients with preoperative LVESVI < 80 ml/m2 have, however, a five-year survival of 79%. This value decreases, however, to 72% and 67% for patients with LVESVI between 80-120 ml/m2, and greater then 120 ml/m2 respectively.
Preoperative ejection fraction (EF) is also an important determinant for survival. Patients that underwent the SAVER operation and had a preoperative EF > 30% had a 77% 5-year survival while patients with an EF < 30% had a 64% survival [7].
Patients younger then 70 years of age had a 5-year survival of 70%, while older patients had only a 59% actuarial survival [4].
Overall the freedom from readmission to the hospital for heart failure after the SAVER operation is 78% at five years [4]. Preoperative NYHA class improves from an average of 2.9 with 67% of patients in class III and IV to 1.7 with only 15% of patients in class III and IV [4].
In the current case presentation, the patient was severely symptomatic preoperatively and had a low ejection fraction. However she had a low LVESVI and was in a favorable age group. Her postoperative showed that she benefited from the SAVER operation.
In conclusion, restoring ventricular shape and function in patients with ischemic cardiomyopathy, especially in patients with anterior myocardial infarction and akinesis, compares favorably with other treatment modalities for this condition regarding early and long-term survival as well as postoperative functional status.
References
1 . Athanasuleas LC, Buckberg GD, Menicanti L, Gharib M, RESTORE Group. Optimizing ventricular shape in anterior restoration. Semin Thor Cardiovasc Surg 2001;13:459-67
2. Menicanti L, DiDonato M. The Dor procedure: what has changed after fifteen years of clinical practice? J Thorac Cardiovasc Surg 2002;124:886-90.
3. Grondin P, Kretz JG, Bical O, Donzeau-Gouge P, Peticlerc R, Campeau L. Natural history of saccular aneurysms of the left ventricle. J Thorac Cardiovasc Surg 1979;77:57-64.
4. Restore Group – SAVER update meeting, Toronto 2004.
5 . Hosepund JD, Bennett LE, Keck BM, Boucek MM, Novick RJ. The registry of the International Society for Heart and Lung Transplanatation: eighteenth official report –2001. J Heart Lung Transplant 2001;20:805-15.
6. Yamaguchi A, Ino T, Adachi H, et al. Left ventricular volume predicts postoperative course in patients with ischemic cardiomyopathy. Ann Thorac Surg 1998;65:434-8.
7. Athanasuleas LC, Stanley AWH, Buckberg GD, Dor V, Di Donato M, Siler W, RESTORE Group. Surgical anterior ventricular endocardial restoration (SAVER) for dilated ischemic cardiomyopathy. Semin Thor Cardiovasc Surg 2001;13:448-58.