ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
A New Sternal Reinforcement Device to Prevent and Treat Sternal Dehiscence
Index
INDEX
Stainless steel wires are currently used for median sternotomy closure in cardiac and general thoracic surgery; this method is safe, effective, and fast. However, the wires can damage and cut bone during respiratory and chest motion. A novel sternal reinforcement device has been designed to prevent wires cutting into the bone.
Stainless steel wires are currently used for median sternotomy closure in cardiac and general thoracic surgery; this method is safe, effective, and fast. However, the wires can damage and cut bone during respiratory and chest motion, leading to instability and chest pain, and may even increase the risk of dehiscence. The incidence of this complication is substantially increased in patients with additional risk factors [1, 2] or when median sternotomy is unintentionally performed on a paramedian line [3]. Standard closure might not be adequate in such patients, and reinforcement of the closure site is usually considered [4]. A novel sternal reinforcement device (DSS) has been designed to prevent wires cutting into the bone; it improves mechanical stability and allows sternal closure with stainless steel wires when bone fracture occurs (Video 1).
Device Description and Surgical Technique
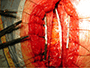
The sternal device (Mikai SpA, Vicenza, Italy) consists of separate clips made of a 0.7 mm thick titanium (Figure 1), sliding one into each other to form two arms placed on both sides of the sternum (Video 2). The horizontal segments of the clips (5 mm wide) can slide into each other for a variable length, thus adapting the length of the support to a patient’s intercostal space size; this allows adjustment of the length of the arm to fit the sternal size. The large vertical grooved arm of the clip (6.4 mm wide) is placed into the intercostals space, premitting no direct contact between the stainless steal wire and the side of the sternum. The clips are available in two sizes: standard (40 mm horizontal and 32.2 mm vertical length) and large (48 mm horizontal and 40.2 mm vertical length) to better adapt to the sternal size
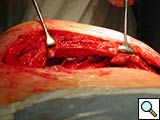
Figure 5. Technique of implantation: the two pliable vertical "fingers" of each unit are bent outwards around the ribs.
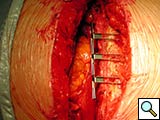
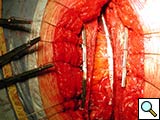
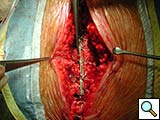
To facilitate insertion of the device, the fascia is dissected from the sternal border with electrocautery, and if necessary, perforating vessels are clipped (Figure 2). Once the fascia is mobilized, the hemisternum is elevated by two hand retractors to optimize exposure. The clips are assembled to form a device of a suitable length (up to 5 clips for each one) and the vertical segments of the clips are inserted into the intercostals spaces (Figures 3, 4). The two pliable vertical “fingers” of each unit are then bent outwards to wrap around the ribs, so that the clips are held firmly in place (Figure 5; Video 3). Re-approximation of the sternum is then achieved by means of single interrupted stainless steel wires. Two to 3 are placed through the manubrium, and the others are placed around the grooved arms of the clips at the level of the intercostal spaces (Figures 6, 7). The fascia, subcutaneous layers, and skin are closed in a routine manner. The extra time required for placement of the reinforcement device decreases with experience, and in our hands is approximately 4 to 6 minutes for each hemisternum.
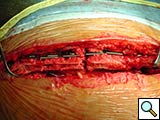
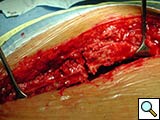
Figure 9. The device (constructed out of four units) is implanted, where the third clip is positioned corresponding to the fractured bone.
Results
Indications for use have been prevention of sternal fracture after sternotomy in patients with severe osteoporosis, when a faulty sternotomy has been performed, in obese patients, or when additional risk factors for wound dehiscence are present. Molina, et al., and other authors have reported a high incidence of sternal dehiscence in obese patients [5]. We also use the device to stabilize the closure site when sternal fracture occurs intraoperatively or is detected postoperatively, requiring reoperation for sternal fixation (Figures 8-10).
Lateral distraction forces are crucial in determining the stability of sternal closures. McGregor, et al., [6] tested forces applied from different directions in cadaveric models and found that less force was required in the lateral direction for separation of the rewired sternal halves. All the current techniques for sternal closure and reinforcement contribute to improve stability; however, the employment of techniques using large plates combined with screws and/or wires may be unsafe in patients with particularly narrow or severely osteoporotic bone. On the other hand, devices such as coils or staples appear easy to use but may not offer adequate support in overweight or “oversized” patients when elevated lateral distraction forces are applied to the sternal closure.
The current device presents some theoretical advantages over current options. It consists of small light modules that can be assembled so that only a part or the full length of sternal halves can be reinforced; the design allows assembling of the clips to form arms of various lengths to adapt to the sternum size; and the wide vertical grooved arm, which is positioned in the intercostals space, avoids direct contact of the wires with the lateral side of the bone and distributes traction forces on a wide surface.
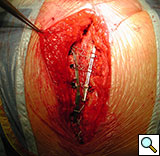
From December 2004 to December 2007, the DSS was implanted in 55 patients in our institution. Of those, 45 patients had at least three preoperative risk factors or experienced bone fracture or paramedian sternotomy detected intraoperatively, and underwent primary sternal reinforcement with the DSS. Of these patients, 75% were diabetic, 50% had chronic obstructive pulmonary disease, and 38% were obese [7]. Only one patient experienced a superficial wound dehiscence. The other 10 patients underwent sternal reconstruction following sternal dehiscence (Figures 11, 12).
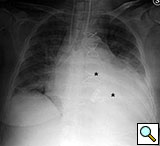
Figure 11. Fourteenth postoperative day image showing sternal wire migration in the caudal sternum segment (*).
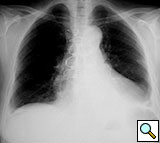
Figure 12. Postoperative sternal reconstruction showing sternal fixation with DSS (2 and 3 units in the right and left hemisternum, respectively
Implantation of the device presents some potential complications, including bleeding due to damage to the internal thoracic vessels during implantation (if not harvested for revascularization), and partial sternal devascularization due to mobilization of the superficial pectoralis fascia, which might be pronounced in patients undergoing CABG surgery with bilateral mammary arteries, thus interfering with wound healing. However, in most patients in whom sternal reinforcement is required, available techniques and materials potentially jeopardize blood supply to the sternum.
In conclusion, our preliminary clinical experience in this selected group of patients at high risk and patients with postoperative wound dehiscence demonstrates that the proposed technique adds little time to the operation, is easy to perform, and carries a low risk of complications.
Disclosure
The first author (JZ) discloses a financial arrangement with the manufacturer of this device.
References
- Ridderstolpe L, Gill H, Granfeldt H, Ahlfeldt H, Rutberg H. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg 2001;20:1168-75.
- Jonkers D, Elenbaas T, Terporten P, Nieman F, Stobberingh E. Prevalence of 90-days postoperative wound infections after cardiac surgery. Eur J Cardiothorac Surg 2003;23:97-102.
- Zeitani J, Penta de Peppo A, Moscarelli M, et al. Influence of sternal size and inadvertent paramedian sternotomy on stability of the closure site: a clinical and mechanical study. J Thorac Cardiovasc Surg 2006;132:38-42.
- Robicsek F, Daugherty HK, Cook JW. The prevention and treatment of sternum separation following open-heart surgery. J Thorac Cardiovasc Surg 1977;73:267-8.
- Molina JE, Lew RS, Hyland KJ. Postoperative sternal dehiscence in obese patients: incidence and prevention. Ann Thorac Surg 2004;78:912-7.
- McGregor WE, Trimble DR, Farkas KM, Magovern JA. Mechanical analysis of midline sternotomy wound closure. J Thorac Cardiovasc Surg 1999;117:1144–49.
- Zeitani J, Penta de Peppo A, Bianco A, et al. Performance of a novel sternal synthesis device after median and faulty sternotomy: mechanical test and early clinical experience. Ann Thorac Surg 2008;85:287-93.