ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Redo Valve-Sparing Ascending Aorta and Total Arch Replacement in a Young Patient With Loeys-Dietz Syndrome: A Challenging Procedure
Marin Cuartas, Mateo; Rendon, Juan Camilo; Jaramillo, Juan Santiago (2017): Redo Valve-Sparing Ascending Aorta and Total Arch Replacement in a Young Patient With Loeys-Dietz Syndrome: A Challenging Procedure.
CTSNet, Inc. https://doi.org/10.25373/ctsnet.5561749
Retrieved: 20:05, Nov 14, 2017 (GMT)
The authors present a successful surgical treatment in a 14-year-old girl with Loeys-Dietz syndrome; a redo aortic arch and ascending aorta replacement with a valve-sparing operation was performed. Special considerations were taken, and a well-established surgical plan was designed to reduce risk and improve outcomes in this complex aortic redo operation.
Case Report
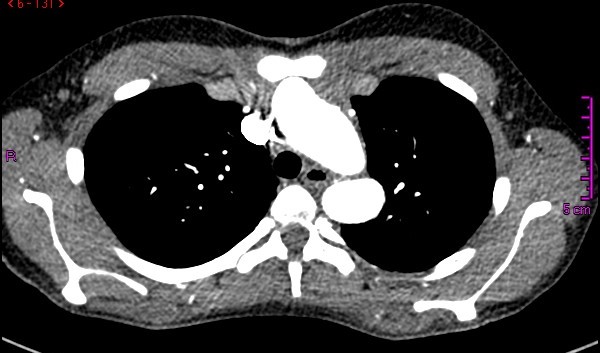
Figure 1. Cross-sectional CT angiogram view of the thorax, where a dilated aortic arch near the origin of the supraaortic vessels is observed in close proximity to the sternum.
A 14-year-old female patient with clinical features of Loeys-Dietz syndrome presented to the authors’ institution with new onset atypical chest pain. The patient related a prior surgical correction of an ascending aorta aneurysm at 18 months of age, in addition to a sibling who had an ascending aorta aneurysm surgically corrected in early childhood. Transthoracic echocardiography showed a tricuspid aortic valve with mild insufficiency, dilation of the aortic root, and dilation at proximal and distal anastomosis of the ascending aorta Dacron tube, without stenosis. Supraaortic vessels showed a tortuous and dilated origin. Given the technical difficulty for surgical correction, computed tomography (CT) angiography of the thoracic aorta was requested with 3D reconstruction for surgery planning. This demonstrated postsurgical inflammatory changes, supraaortic vessels only 2 mm from the sternum, aneurysmal dilatation of the aortic root, ascending aorta, and aortic arch, tortuosity and dilatation of supraaortic vessels, aberrant right subclavian artery, and a normal descending aorta (Figures 1 and 2). A surgical plan was established and was followed throughout the operation by the entire team:
- Hemodynamic monitoring was performed with an internal jugular central venous catheter and bilateral radial and left femoral artery lines.
- Arterial and venous cannulation for cardiopulmonary bypass (CPB) was performed through the right femoral artery and vein, respectively.
- Systemic heparinization.
- CPB was begun.
- Systemic cooling to 26°C.
- Median sternotomy, adhesion release, and supraaortic vessels dissection.
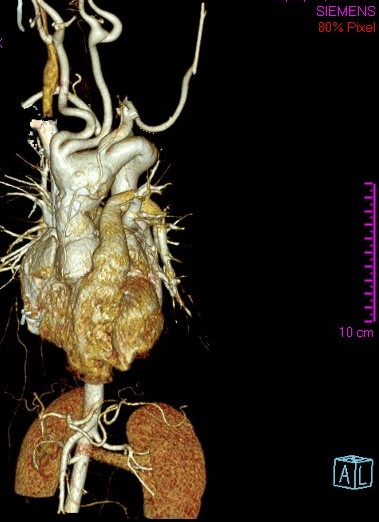
Figure 2. Three-dimensional CT reconstruction showing aneurysmal dilatation of the aortic root, ascending aorta, and aortic arch, tortuosity of supraaortic vessels, and aberrant right subclavian artery.
Thereafter, aortotomy was performed under moderate hypothermia, and cold Del Nido cardioplegia was administered through the coronary ostia. After descending aorta endoclamping with a Foley catheter, pathologic aortic tissues were transected and the aortic arch was inspected under direct vision. Four retrograde arterial cannulas attached to “Y” connectors in the arterial line were then placed into each supraaortic artery. Subsequently, a Tirone-David V procedure and aortic arch replacement were successful performed. The patient was transferred to the intensive care unit where she recovered uneventfully with early extubation, and she was discharged from the hospital after 10 days.
Discussion
Loeys-Dietz syndrome (LDS) is an autosomal dominant aortopathy with an aggressive and lethal behavior due to aortic aneurysms and dissections, which tend to rupture even at small to intermediate diameters (1). An aortic root diameter greater than 4 cm in an adult LDS patient is considered the threshold for surgical intervention (2). Rapid growth of the aortic aneurysm or the presence of symptoms such as chest pain are indications for surgical management. Surgical preservation of the native aortic valve should be attempted in order to offer the hemodynamic advantages of a native heart valve and to prevent anticoagulation. A suitable length for the Dacron graft in a growing child is difficult to determine; the patient’s age and estimated physical growth have to be taken into consideration. Imaging surveillance after surgery is the recommended practice because of the likely occurrence of future aortic syndromes (2-4).
Redo heart surgery is technically difficult and carries a high surgical risk. Difficult access to the heart due to postinflamatory changes increases the possibility of serious injuries during chest opening. Operative times, bleeding, and infection rates are also higher. The success of redo cardiac surgeries depends on the organization and communication of the heart team. In this way, it is of absolute importance to develop an operative plan that is shared throughout the surgical team. It describes the procedure step-by-step in an orderly way, provides technical details, and assigns clear roles, as well as contemplates possible complications with their corresponding solution.
Peripheral cannulation can be very useful in patients with significant chest adhesive processes. It is advisable to use a catheter that comes directly from the arterial peripheral cannula to be inserted distally into the superficial femoral artery, thus ensuring distal perfusion. Once the patient has been cannulated, CPB is initiated before sternum opening. The use of assisted venous drainage with a universal vacuum system allows emptying of the heart, thus achieving a distance between critical structures and the sternum during sternotomy.
Hypothermic cerebral and mesenteric CPB favors tissue protection allowing longer aortic cross-clamping times in a safe manner in surgeries of high technical complexity, which require a depurated surgical technique for the vascular anastomosis avoiding postoperative bleeding (5).
References
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-β receptor. N Engl J Med. 2006;355(8):788-798.
- Williams JA, Loeys BL, Nwakanma LU, et al. Early surgical experience with Loeys-Dietz: a new syndrome of aggressive thoracic aortic aneurysm disease. Ann Thorac Surg. 2007;83(2):S757-S763.
- Ozker E, Vuran C, Saritas B, Türköz R. Valve-sparing replacement of the ascending aorta and aortic arch in a child with Loeys-Dietz syndrome. Eur J Cardiothorac Surg. 2012;41(5):1184-1185.
- Everitt MD, Pinto N, Hawkins JA, Mitchell MB, Kouretas PC, Yetman AT. Cardiovascular surgery in children with Marfan syndrome or Loeys-Dietz syndrome. J Thorac Cardiovasc Surg. 2009;137(6):1327-1332.
- Cohn LH. Evolution of redo cardiac surgery: review of personal experience. J Card Surg. 2004;19(4):320-324.





Comments