ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Surgical Treatment for Early Stage Thymomas: Approach and Technique
Early stage thymomas are rare, indolent tumors of the thymus gland. Thymomas may develop at any age, but are most common between the ages of 35 and 70 years. Distribution between genders tends to be fairly equal, with a slight female predominance among older age groups (1). Approximately 30-45% of patients with thymomas will have symptoms of myasthenia gravis (MG); however, only 10-15% of patients with MG are found to have a thymoma (2). One-third of patients diagnosed with thymomas are asymptomatic at the time of diagnosis, and the thymoma is discovered incidentally on imaging for other causes. Forty percent of patients present with local symptoms, most commonly chest pain, cough, and shortness of breath from external compression of adjacent airways. An additional 30-40% of patients present with systemic syndromes, including MG, pure red cell aplasia, and hypogammaglobulinemia (3).
Diagnosis
Although rare, thymomas are the most frequently occurring tumors of the anterior mediastinum. They account for approximately 35-50% of all anterior mediastinal masses (1, 3). Other commonly occurring tumors of the anterior mediastinum include lymphoma (25%), thyroid and parathyroid tumors (15%), benign teratoma (10%), malignant germ cell tumors (10%), and benign thymic lesions (5%) (4). It is essential to differentiate thymic malignancies from other anterior mediastinal masses prior to treatment. Evaluation of an anterior mediastinal mass should begin with a detailed history and physical. Age and gender are particularly important, as specific lesions tend to occur more frequently in certain demographic groups. The presence of local or systemic symptoms may also help differentiate the type of lesion. CT imaging is the preferred imaging modality, as the appearance of a mass may be highly suggestive of a particular diagnosis (Figure 1). MRI can be helpful in distinguishing solid and cystic lesions. CT-PET is not recommended for the routine evaluation of a thymoma, but is useful in staging lymphoma and evaluating response to therapy. Laboratory studies are an important adjunct as well. Elevated levels of α-fetoprotein (α-FP) and β-human chorionic gonadotropin (β-HCG) are diagnostic of non-seminomatous germ cell tumors, while elevated lactate dehydrogenase (LDH) is a common finding in lymphoma (4).
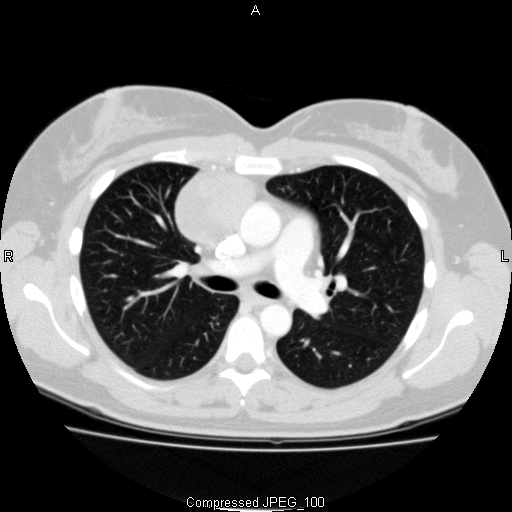
Figure 1: CT image demonstrating a well-encapsulated
anterior mediastinal mass representative of an early stage
thymoma.
Staging
No official staging system exists for thymic malignancies. However, the most widely accepted classification system is one that was first proposed by Masaoka et al. in 1981, and later modified by Koga and colleagues in 1994 (5, 6). The Masaoka-Koga staging system is largely based upon the extent of local invasion of the primary tumor, since nodal involvement is relatively infrequent. Tumor extension, as seen on gross and microscopic examination, post-resection has proven to be a good indicator of predicted survival (7). The International Thymic Malignancy Interest Group (ITMIG) has adopted the Masaoka-Koga staging system for thymic malignancies, and added additional definition of details as outlined in Table 1 (8). Data from multiple large studies show that approximately 40% of thymomas present as stage I tumors, 25% as stage II, 25% as stage III, 10% as stage IVa, and 1-2% as stage IVb (2).
Treatment
Complete resection is the cornerstone of treatment, and has been shown to be effective in achieving high cure rates (9-12). For this reason, en bloc resection of the entire thymus gland and surrounding mediastinal areolar tissue is the standard of care in most centers today (Figure 2). Approximately 50% of thymomas are found to have invaded mediastinal tissue by the time of diagnosis. Pleural invasion is most common, followed by pulmonary and pericardial invasion, as well as involvement of the innominate vein, superior vena cava, or phrenic nerve (3). Extension into the aorta, pulmonary artery, or chest wall may also occur. It may be necessary to remove and reconstruct major vital structures (such as the superior vena cava, innominate vein, or aorta) in order to achieve a complete resection. If one phrenic nerve is involved, it should be resected. Only in MG patients with severe symptoms and significantly limited pulmonary reserve, the phrenic nerve should not be resected (8). Careful preoperative planning is crucial as complete resection has been associated with improved survival. Ten-year survival rates after complete resection are 90%, 70%, 55%, and 35% for stages I, II, III, and IV, respectively (3). Recurrence rates after complete resection for stages I, II, and III are 3%, 16%, and 26%, respectively. The mean time to recurrence has been reported as 10 years for stage I and 3 years for stages II-IV (3).
Preoperative chemotherapy has shown promising results in stage III and IV thymomas. Several large series have reported increased R0 resection rates after preoperative chemotherapy (72%), as opposed to surgery alone (50% for stage III, 25% for stage IV) (3). Therefore, preoperative chemotherapy should be considered for clinical stage III and IVa tumors.
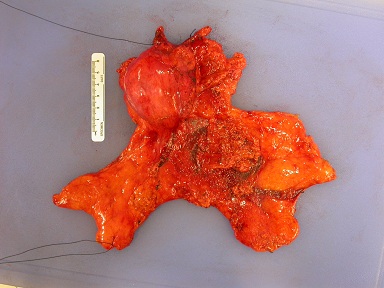
Figure 2: Complete resection of thymus gland without
disruption of the thymic capsule.
Role of Lymph Node Dissection
Lymph node dissection is a common practice in oncologic surgery, as diagnosis of clinically undetectable lymph node metastases impacts prognosis and helps guide adjuvant therapy. However, there is no consensus on lymph node dissection for thymic malignancies. As previously described, the Masaoka-Koga staging system emphasizes local invasion of the primary tumor, as this has been shown to be prognostic and more common than lymph or hematogenous spread. While this is true in the case of thymoma, thymic carcinoma and carcinoid tumors have a higher rate of lymph node metastasis. A study by Kondo and Monden comparing the incidence of lymph node metastasis among thymic epithelial tumors found that only 19 cases (1.8%) of 1,064 thymomas metastasized to the lymph nodes; while 49 (26.8%) of 183 thymic carcinomas and 11 (27.5%) of 40 thymic carcinoids were associated with positive lymph nodes (13). Anterior mediastinal lymph nodes were most frequently involved, and lymph node involvement without primary tumor invasion into adjacent organs was rare. The authors noted a significant difference in the 5-year survival rate among thymomas and thymic carcinomas with and without lymph node metastasis (95.6% if node-negative vs. 61.5% if node-positive thymoma; 56% if node-negative vs. 42.1% if node-positive thymic carcinoma, p<0.0001) (13).
A recent study by Weksler and colleagues examining 2,227 thymoma patients entered into the Surveillance, Epidemiology, and End Results (SEER) database found that only 19.8% of thymectomies performed for thymoma included pathologic analysis of lymph nodes (14). The incidence of lymph node metastases among thymoma patients was higher (13.3%) than that reported by Kondo and Monden (1.8%), although this finding may be related to the inclusion of more patients with advanced stage disease. The presence of lymph node metastases was an independent predictor of shortened disease-specific survival in multivariate analysis.
In 2014, ITMIG and the International Association for the Study of Lung Cancer published a proposed lymph node map for TNM staging of thymic tumors (15). The proposed map divides the mediastinum into twp regions: anterior and deep. The anterior region contains the perithymic nodes and is defined as the space anterior to the pericardium, inferior to the hyoid bone and between the mediastinal pleurae. Positive anterior lymph nodes are defined as N1 disease. The deep region includes the paratracheal and aortopulmonary nodes. It is located between the anterior region and the esophagus, and between the pulmonary hila. Positive lymph nodes in this region are defined as N2 disease. The new proposal classifies N1 involvement as stage IVa disease, while N2 involvement is stage IVb disease. Any positive lymph node outside these two regions is considered M1 disease (stage IVb). Although empiric, ITMIG strongly encourages surgeons to resect anterior mediastinal nodes for localized thymomas, resect anterior mediastinal nodes and sample intrathoracic (deep region) nodes for invasive thymomas, and resect both anterior and deep regional nodes for thymic carcinoma (15).
Surgical Techniques
Sternotomy
The traditional surgical approach for a thymectomy has been through a median sternotomy (3). This approach provides the best exposure and access to all four lobes of the thymus gland, as well as the pericardial fat and areolar tissue of the mediastinum. The boundaries of a complete resection extend from: the left and right phrenic nerves laterally, the sternum anteriorly and pericardium posteriorly, and the subxiphoid space and diaphragm inferiorly, and the neck superiorly (1). Any adherent structure should be resected en bloc with the specimen. Careful tissue handling is of great importance and care must be taken not to rupture the tumor capsule (16).
Thymectomy via sternotomy is performed under general anesthesia using a double-lumen endotracheal tube. Use of a double-lumen tube allows for lung isolation, so that both pleural spaces can be thoroughly evaluated. The patient is placed supine on the operating table with both arms tucked. After performing a median sternotomy, the pleura of both hemithoraces is opened along the pericardium, taking care not to injure the phrenic nerves. The pleural surfaces should be carefully inspected for tumor involvement. The thymus gland is then resected superiorly off the pericardium. Fatty tissue adjacent to the diaphragm should be taken as part of the specimen. The dissection is carried along the right side of the aortopulmonary window as well as to the left of the aorta. Branches from the innominate vein are ligated. The thymus gland is then followed superiorly into the neck, where the horns of the gland are usually the last portion to be dissected (3, 17).
Once the specimen is removed from the patient, it can be difficult to determine the correct orientation as well as any areas that may have been of concern for close or disrupted margins. For this reason, it is recommended that areas of concern be marked at the time of dissection to ensure proper identification and microscopic examination by the pathologist (16). These areas should be marked with a secure stitch to prevent disruption of the stitch during handling of the specimen. At the same time, the specimen is marked with a stitch, the adjacent area in the operative field should be marked with a clip so that it can be identified if radiation is indicated postoperatively. In addition to marking areas of concern, ITMIG recommends certain standard areas be marked routinely. These areas include the surface of the specimen adjacent to the pericardium, innominate vein, superior vena cava (SVC), and right and left pleural surfaces. Marking areas adjacent to the SVC and pleura may not be possible in cases of small thymomas. Areas of concern should be directly communicated to the pathologist. It may be useful to orient the specimen on top of a mediastinal board or a diagram of the mediastinum (16).
Minimally Invasive Approaches
Minimally invasive thymectomy can be done through a variety of approaches, including transcervical, extended transcervical, video-assisted thoracoscopic, and robotic approaches (1,18). Minimally invasive resection refers to the surgical approach and type of incision. It does not refer to a limited dissection, and should not compromise or change the actual resection relative to an open approach. The ability to achieve a complete resection remains the primary goal. Incomplete resection and debulking are not acceptable for minimally invasive resections, and conversion to open surgery is mandatory if this is the case.
Certain patients will not be suitable candidates for a minimally invasive approach. Patients who are morbidly obese or who have limited neck extension are not suitable candidates for transcervical excision, as access to the mediastinum from the head of the operating table is significantly compromised (19, 20). Prior median sternotomy or radiation to the anterior mediastinum are relative contraindications to minimally invasive approaches as scar tissue makes dissection very difficult (19). The size of the tumor should also be considered when deciding on the appropriate operative approach. Minimally invasive approaches are best suited to small (<5 cm), early stage thymomas that do not invade adjacent vital organs, including the great vessels, heart, and trachea (19-21).
It is best to approach a minimally invasive resection systematically. Before starting any dissection, the thymoma should be visualized and assessed for minimally invasive resection (18). Similar to an open approach, a complete resection should include all tissue between both phrenic nerves, between the sternum and pericardium, and from the diaphragm into the neck. If the thymoma is not adherent to any major blood vessels, the phrenic nerve or the sternum, the surgeon may proceed with a minimally invasive resection (18). The thymoma should be resected en bloc with the thymus gland, ideally without exposing the tumor and with minimal handling. Grasping or squeezing the tumor risks rupture of the capsule and increases the risk of pleural dissemination. As in open procedures, any areas of concern for potential tissue disruption ought to be marked during the dissection and communicated to the pathologist. Adjacent mediastinal nodes and any suspicious nodes should be included in the resection specimen. A protective bag is used to remove the specimen through an incision that is large enough so as not to disrupt the tumor capsule during extraction. Every attempt should be made to avoid dragging the specimen across the mediastinum or pleura to prevent dissemination (18).
Video-Assisted Thoracoscopic Surgical (VATS) and Robotic-Assisted Thymectomy
The technique of VATS thymectomy can be performed via a right, left, or bilateral approach. The appropriate approach is determined by the location of the tumor as seen on preoperative CT scan, and by the ability to achieve a complete resection. A double-lumen endotracheal tube should be used for intubation, as lung isolation provides necessary visualization. For a right-sided or centrally located tumor, the patient is placed in a partial left lateral decubitus position (approximately 30o of upward tilt) with the right arm in a swimmer’s position. The right arm should be well padded to prevent an ulnar nerve palsy. It is helpful to place a bump under the right chest to provide access to the axilla. The entire chest should be prepped and draped in the chance that access to the left hemithorax is needed. Typically, a 3-port technique is used. There is some variability in port placement, but the standard position of the thoracoscope is through a 12-mm port in the fourth intercostal space along the posterior axillary line. Two 5-mm instrument ports are then placed under direct visualization: one in the third intercostal space along the midaxillary line, and the other in the sixth intercostal space at the anterior axillary line (17, 21-23).
The right lung is retracted, and the right phrenic nerve is identified. Using electrocautery, the mediastinal pleura is incised parallel to the phrenic nerve. Dissection is continued superiorly to the level of the internal mammary vessels, and then transversely across the superior mediastinum staying anterior to the innominate vein, and traveling as far laterally as visualization allows. The dissection then proceeds lateral to medial, along the inferior border at the pericardial reflection. Once the left pleura and left phrenic nerve are encountered, dissection is carried cephalad parallel to the nerve. The thymus is then mobilized off the underlying pericardium and innominate vein. For left-sided tumors or access to the left chest, the set-up is a mirror image of that described for the right-sided procedure (17, 21-23).
Electrocautery, harmonic scalpel, and LigaSureTM (Covidien, Mansfield, MA, USA) are helpful and complementary tools for dissection. The hook cautery allows for more precise surface-type cautery, and can be used to dissect around vessels. The harmonic scalpel and LigaSureTM are useful for sealing and transecting small vessels. Larger, draining branches to the innominate may need to be clipped and transected. Once the thymus and surrounding fatty tissue have been completely dissected, the specimen is placed in a protective bag and extracted. The specimen should be removed and examined on the back table and oriented as previously described. A small chest tube (16-20Fr) is often placed in the anterior mediastinum.
The introduction of the da VinciTM robotic system (Intuitive Surgical, Sunnyvale, CA, USA) has provided an additional approach to minimally invasive thymectomy. The procedure is performed with the patient under general anesthesia using a double-lumen endotracheal tube for split-lung ventilation. The anterior mediastinum may be approached from the right, left, or bilateral sides, depending upon the extent and location of the mass. Patients are positioned supine. However, if a unilateral approach is being used, slight elevation of the operative side may facilitate movement of the most cephalad robotic arm. The patient’s ipsilateral arm should be positioned along his or her side, as far back as possible to provide enough space for the more inferior robotic arms (Figure 3). The robotic technique most frequently uses three ports: one camera port and two working ports. The positions of the ports may vary slightly. The camera port is placed first, and is positioned along the anterior axillary line in the fifth intercostal space. Additional ports are placed along the anterior axillary line at the third intercostal space, and along the midclavicular line at the fifth or sixth intercostal space. Carbon dioxide insufflation of the mediastinum is used at a pressure of 4-8 mmHg to enlarge the operative field. As previously described, the surgeon should avoid handling the specimen as much as possible, so as to prevent rupture of the capsule. The resection then proceeds in a similar fashion as previously described for a VATS approach (24-26).
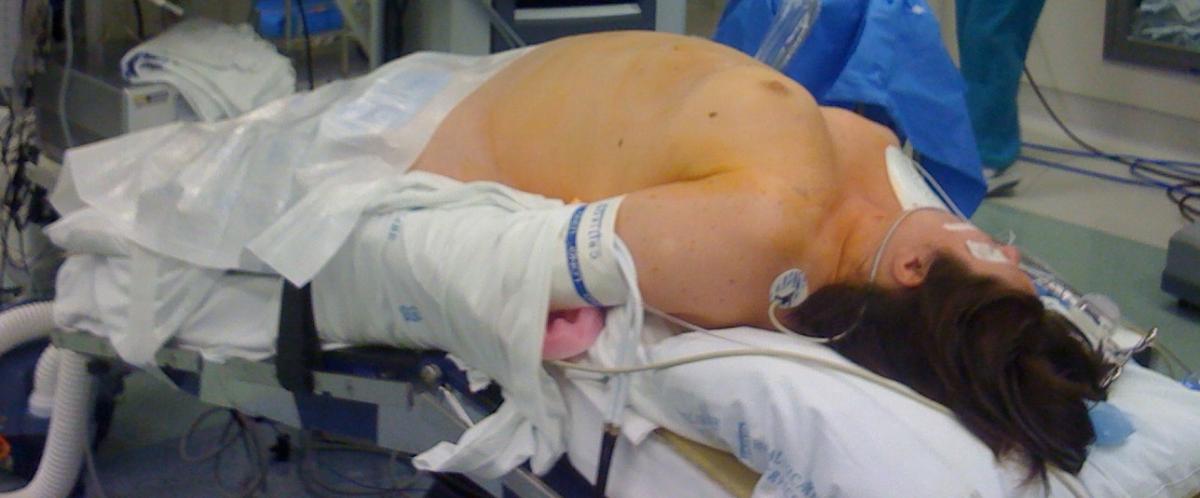
Figure 3: Positioning for robotic thymectomy. The patient’s arm on the
operative side can be tucked or positioned laterally on an arm board based
on surgeon preference.
Extended Transcervical Thymectomy
Indications for extended transcervical thymectomy include myasthenia gravis, thymic cysts, and small, noninvasive thymomas (19). For this technique, general anesthesia through a single- or double-lumen endotracheal tube is used. The patient is positioned supine with an inflatable bag placed behind the shoulders to extend the neck. The patient’s head may be turned to the left to further improve access to the mediastinum. The entire neck and chest are prepped and draped so that the sternum is accessible should conversion to a sternotomy be required. It is recommended that the surgeon wear a headlight to improve visualization. A 30o thoracoscope is also used to provide illumination and visualization within the mediastinum (19, 20).
A 5-cm transverse, curvilinear skin incision is made 2 cm above the sternal notch, and between the sternocleidomastoid muscles. Subplatysmal flaps are created superiorly to the level of the thyroid cartilage, and inferiorly to the sternal notch. The strap muscles are divided in the midline, down to the sternal notch. The superior poles of the thymus are located behind the strap muscles. Often the left pole is identified first, as it tends to be larger. The superior poles have the appearance of encapsulated fat, and can be bluntly dissected off the strap muscles with the use of a Kittner dissector. The dissection is carried cephalad and laterally until the thyrothymic ligament is reached. The ligament is then divided between ligatures. The tails of the suture are left long on the thymic side, as they can be used to place traction on the gland during dissection into the superior mediastinum. A plane is developed posterior to the sternum using blunt dissection with a finger. A self-retaining, self-elevating retractor is then placed. Deflation of the bag beneath the shoulders helps open up the anterior mediastinum. Army-Navy retractors may be placed at the top corners of the incision and tied to the retractor using Penrose drains to further expose the operative field. Fish hooks may also be used to retract the edges of the incision for better exposure (Figure 4) (19, 20).
The thymus is then retracted forward and upward, using the silk sutures placed on the superior poles. The thymus is dissected into the superior mediastinum using a combination of blunt, sharp, and cautery dissection. The thymic veins will be encountered posterior to the gland, where they drain into the innominate vein. These are sequentially suture ligated and divided, as clips may become dislodged with passage of instruments through this area. The dissection of the thymus and the surrounding fatty tissue is then continued progressively deeper into the mediastinum. A sponge stick can help facilitate blunt dissection down to the diaphragm and laterally to the pleural reflections and phrenic nerves. A thoracoscope introduced through the neck incision may facilitate visualization during this portion of the procedure. Holding ventilation intermittently may also facilitate exposure. Small branches from the internal mammary may be encountered and divided between clips. The thymus is removed once it has been dissected off the pericardium. Prior to wound closure, air can be evacuated from the pleural space with a red rubber catheter, while holding a sustained positive pressure breath. Chest tubes are not routinely left in place (19, 20).
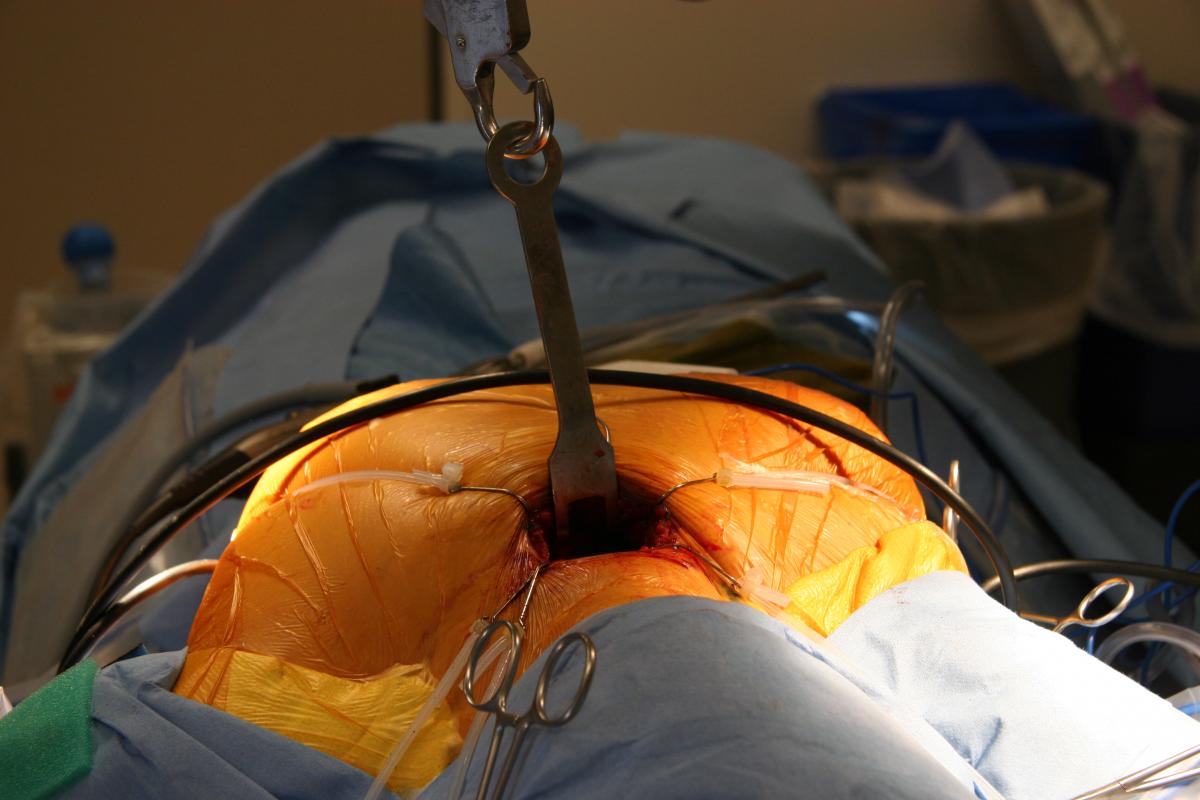
Figure 4: Standard set-up for extended transcervical thymectomy.
Results
Several studies have been published comparing different minimally invasive approaches to open resection for thymic malignancies. A summary of the most recent studies is listed in Table 2. No consensus exists as to which approach is superior. Various authors have shown shorter operative times, less intraoperative blood loss, decreased postoperative pleural drainage, and shorter hospital stays using a minimally invasive approach. However, these studies tend to be small and retrospective in nature. Ultimately, the best approach should be determined by patient and tumor characteristics, the surgeon’s experience and comfort level, and the ability to adhere to the tenets of oncologic surgery.
References
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chinese medical journal 2013; 126: 2186-2191.
- Detterbeck FC, Parsons AM. Management of stage I and II thymoma. Thoracic surgery clinics 2011; 21: 59-67, vi-vii.
- Detterbeck F, Parsons A. Thymic tumors: a review of current diagnosis, classification, and treatment. In: GA Patterson JD, A Lerut, JD Luketich, TW Rice, FG Pearson editor. Thoracic and esophageal surgery, 3rd ed. Philadelphia: Elsevier; 2008. p. 1589-1614.
- Carter BW, Marom EM, Detterbeck FC. Approaching the patient with an anterior mediastinal mass: a guide for clinicians. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2014; 9: S102-109.
- Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981; 48: 2485-2492.
- Koga K, Matsuno Y, Noguchi M, Mukai K, Asamura H, Goya T, Shimosato Y. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathology international 1994; 44: 359-367.
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. The Annals of thoracic surgery 2003; 76: 878-884; discussion 884-875.
- Detterbeck FC, Nicholson AG, Kondo K, Van Schil P, Moran C. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2011; 6: S1710-1716.
- Blumberg D, Port JL, Weksler B, Delgado R, Rosai J, Bains MS, Ginsberg RJ, Martini N, McCormack PM, Rusch V, et al. Thymoma: a multivariate analysis of factors predicting survival. The Annals of thoracic surgery 1995; 60: 908-913; discussion 914.
- Maggi G, Giaccone G, Donadio M, Ciuffreda L, Dalesio O, Leria G, Trifiletti G, Casadio C, Palestro G, Mancuso M, et al. Thymomas. A review of 169 cases, with particular reference to results of surgical treatment. Cancer 1986; 58: 765-776.
- Regnard JF, Magdeleinat P, Dromer C, Dulmet E, de Montpreville V, Levi JF, Levasseur P. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. The Journal of thoracic and cardiovascular surgery 1996; 112: 376-384.
- Yagi K, Hirata T, Fukuse T, Yokomise H, Inui K, Ike O, Mizuno H, Aoki M, Hitomi S, Wada H. Surgical treatment for invasive thymoma, especially when the superior vena cava is invaded. The Annals of thoracic surgery 1996; 61: 521-524.
- Kondo K, Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. The Annals of thoracic surgery 2003; 76: 1859-1864; discussion 1864-1855.
- Weksler B, Pennathur A, Sullivan JL, Nason KS. Resection of thymoma should include nodal sampling. The Journal of thoracic and cardiovascular surgery 2015; 149: 737-742.
- Bhora FY, Chen DJ, Detterbeck FC, Asamura H, Falkson C, Filosso PL, Giaccone G, Huang J, Kim J, Kondo K, Lucchi M, Marino M, Marom EM, Nicholson AG, Okumura M, Ruffini E, Van Schil P, Staging, Prognostic Factors C, Advisory B. The ITMIG/IASLC Thymic Epithelial Tumors Staging Project: A Proposed Lymph Node Map for Thymic Epithelial Tumors in the Forthcoming 8th Edition of the TNM Classification of Malignant Tumors. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2014; 9: S88-96.
- Detterbeck FC, Moran C, Huang J, Suster S, Walsh G, Kaiser L, Wick M. Which way is up? Policies and procedures for surgeons and pathologists regarding resection specimens of thymic malignancy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2011; 6: S1730-1738.
- Jurado J, Javidfar J, Newmark A, Lavelle M, Bacchetta M, Gorenstein L, D'Ovidio F, Ginsburg ME, Sonett JR. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. The Annals of thoracic surgery 2012; 94: 974-981; discussion 981-972.
- Toker A, Sonett J, Zielinski M, Rea F, Tomulescu V, Detterbeck FC. Standard terms, definitions, and policies for minimally invasive resection of thymoma. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2011; 6: S1739-1742.
- Shrager JB. Extended transcervical thymectomy: the ultimate minimally invasive approach. The Annals of thoracic surgery 2010; 89: S2128-2134.
- Komanapalli CB, Cohen JI, Sukumar MS. Video-assisted extended transcervical thymectomy. Innovations 2007; 2: 90-94.
- Liu TJ, Lin MW, Hsieh MS, Kao MW, Chen KC, Chang CC, Kuo SW, Huang PM, Hsu HH, Chen JS, Lai HS, Lee JM. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Annals of surgical oncology 2014; 21: 322-328.
- Whitson BA, Andrade RS, Mitiek MO, D'Cunha J, Maddaus MA. Thoracoscopic thymectomy: technical pearls to a 21st century approach. Journal of thoracic disease 2013; 5: 129-134.
- Ye B, Tantai JC, Ge XX, Li W, Feng J, Cheng M, Shi JX, Zhao H. Surgical techniques for early-stage thymoma: Video-assisted thoracoscopic thymectomy versus transsternal thymectomy. The Journal of thoracic and cardiovascular surgery 2013.
- Ye B, Tantai JC, Li W, Ge XX, Feng J, Cheng M, Zhao H. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World journal of surgical oncology 2013; 11: 157.
- Freeman RK, Ascioti AJ, Van Woerkom JM, Vyverberg A, Robison RJ. Long-term follow-up after robotic thymectomy for nonthymomatous myasthenia gravis. The Annals of thoracic surgery 2011; 92: 1018-1022; discussion 1022-1013.
- Ismail M, Swierzy M, Ruckert JC. State of the art of robotic thymectomy. World journal of surgery 2013; 37: 2740-2746.




