Introduction
Replacement of the aortic valve remains an effective treatment of severe aortic stenosis (AS). With the aging population, aortic stenosis will be an increasing disease entity to be treated by cardiac surgeons. Although open surgical aortic valve replacement has been the gold standard treatment for severe AS, many patients do not have this option due to severe co-morbidities. Although some of these patients may be candidates for balloon valvuloplasty, this procedure has not offered any improvement in mortality [1]. Catheter-based aortic valve implantation has shown promising results in providing treatment options for patients with severe AS who are poor open surgical candidates. For cardiac surgeons to be involved and to play an important role in the care of these patients, comfort with this methodology will be imperative in the future. This review will provide a brief introduction into catheter-based approaches for the treatment of severe AS, with a focus on the operative techniques.
Three catheter-based bioprosthetic aortic valves have been used in human trials: Cribier-Edwards valve (Edwards Lifesciences Corporation, Irvine, CA), Medtronic CoreValve (Medtronic Inc, Minneapolis, Minnesota), and the Edwards Sapien valve (Edwards Lifesciences Corporation, Irvine, CA). They have been implanted in humans utilizing three basic approaches: 1) transapical placement through a direct apical puncture; 2) retrograde transfemoral approach through the femoral artery, and; 3) antegrade transfemoral approach through the femoral vein. Transaxillary and transcarotid approaches have also been utilized successfully for percutaneous valve implantation, but these techniques require advanced training and have been employed in specific situations under select hands [2]
Transapical approach
In this technique, the bioprosthetic valve is delivered through a direct puncture into the left ventricular apex, accessed via a small anterolateral thoracotomy. The left ventricular cavity is accessed via a needle puncture into the apex, serially dilated to enable employment of the valve delivery system. The disadvantages of this method include bleeding complications from the apical puncture, pericardial effusion with potential tamponade, pseudoaneurysm formation, pneumothorax, poor ventricular remodeling, ventricular arrhythmias, possible damage to the mitral subannular apparatus, and coronary artery injury. The primary advantage is that it allows a direct access to the aortic valve, and is a great alternative in patients with severe peripheral vascular disease, inaccessible tortuous iliac artery disease, or those with a history of extensive major vascular operations.
Transcatheter retrograde approach
In this technique, the femoral artery is accessed through a groin cut-down, and the valve is delivered in a retrograde fashion through a sheath placed in the femoral artery. The main advantage of this technique is greater technical ease, and less invasiveness than the transapical approach. This method has been associated with aortofemoral artery injury/ rupture, and carries a potential risk of stroke as the delivery involves crossing the aortic arch.
Percutaneous antegrade approach
This method has been studied in human trials in the past, but is no longer being used. The femoral vein is accessed and the valve is delivered in the aortic position by traversing the atrial septum and the mitral valve. The main advantage is that it can accommodate a large catheter sheath, still be utilized in patients with severe peripheral arterial vascular disease, and avoid any aortofemoral arterial injury. The disadvantages include greater technical difficulty with a more circuitous path to deploy the bioprosthetic valve and potential injury to the mitral valve and its subannular apparatus. This approach is not further discussed here given it is no longer in use in human trials.
Preoperative Work-Up
This should include a complete blood count, coagulation parameters, electrolytes, renal and hepatic panels, electrocardiogram, and chest x ray. Cardiac catheterization is important for risk stratification and to study for any treatable coronary disease, along with evaluating left sided hemodynamics. Every patient should at least get a transthoracic echocardiogram, and possibly a transesophageal echocardiogram if warranted based on the transthoracic findings. Anatomy of the aortic valve annulus and aortic root apparatus must be carefully evaluated in order to choose the appropriate size of the aortic valve prosthesis. Multislice CT angiogram of the chest, abdomen and pelvis, along with M2S reconstruction is critical in planning for the type of approach and intraoperative modifications.
Operation
For optimal safety and results, a hybrid operating room with sophisticated fixed imaging is essential. This includes having the facilities to perform angiographic imaging, provide cardiac anesthesia with transesophageal echocardiography, access to all preoperative diagnostic imaging, and having the ability to efficiently convert to an open operation with cardiopulmonary bypass. Therefore, this requires a multi-disciplinary effort involving cardiovascular surgery, anesthesiology, interventional cardiology, and perfusion team for potential cardiopulmonary bypass. In the United States, only the Edwards Sapien system is available as part of an FDA study (PARTNER trial). Hence, the experience at our center has been primarily with the Edwards aortic bioprosthetic Sapien valve. The implantation technique practiced at our institution will be described. Implantation of the CoreValve system is described elsewhere [3].
Transfemoral retrograde aortic valve implantation
Patient is placed in a supine position with both arms tucked. Temperature sensing foley catheter, radial arterial line and a central venous line with pulmonary artery catheter are placed. The intubated patient is prepped from neck down to the knees, and draped in standard fashion. Transesophageal echocardiography probe is introduced to obtain a preoperative evaluation, and left in place. All patients receive antibiotic prophylaxis and heparin (100 units/kilogram).
In the contralateral groin, access to the femoral artery is obtained percutaneously. A 6 French sheath is introduced into the femoral artery and a pigtail catheter is placed in the ascending aorta to obtain angiographic views of the aortic valve and aortic root for valve deployment. Also, femoral vein access is obtained percutaneously with a 7 French sheath, and a transvenous pacing wire is introduced into the right ventricle.
A cut-down is then performed in the ipsilateral groin and the femoral artery is exposed. Using a 18 gauge needle, wire access is achieved in the femoral artery using Seldinger technique. A 14 Fr sheath is then placed in the femoral artery. Using a straight wire through an AL1 guide catheter (Boston Scientific Corporation, Massachusetts), the stenotic aortic valve is crossed and images are obtained to assess the left ventricular outflow tract, the native aortic valve and its relation to the coronary ostia. The AL1 guide catheter is then advanced into the left ventricle and the straight wire is then exchanged for a stiff wire such as an Extra-stiff Amplatz (Cook Medical, Indiana) or Lunderquist guidewire (Cook Medical, Indiana). Next, a balloon aortic valvuloplasty (BAV) is performed with the use of a Tyshak 22 mm balloon (B. Braun Medical Inc., Pennsylvania) to facilitate crossing of the native stenotic valve with the Edwards Sapien valve (Figure 1). BAV is performed during a short period of rapid ventricular pacing to minimize transvalvular flow and potential embolization. It is critical to stabilize hemodynamics after the valvuloplasty due to hypotension from rapid pacing and from the ensuing aortic insufficiency.
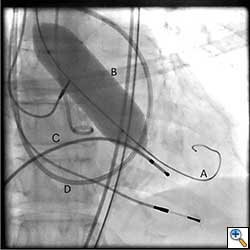
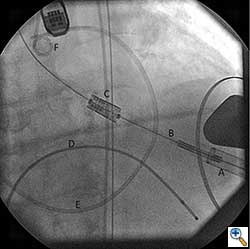
The Sapien valve is a balloon expandable platform and it comes in two sizes, 23 mm and 26 mm. The valve is prepared and mounted onto a sheath system for implantation on the back table. The femoral arterial access is serially dilated to accommodate a 22 French (for 23 mm Sapien valve) or a 24 French (for 26 mm Sapien valve) sheath. The balloon mounted valve is then advanced in a retrograde fashion into the aortic valve position using angiographic and echocardiographic guidance (Figure 2). It is important to visualize the position of the valve using at least 2 different angles. For the transfemoral approach, the recommended positioning of the prosthesis is 60%-40%, which is 60% of the prosthesis should be on the ventricular side of the aortic annulus with 40% of the prosthesis on the aortic side of annulus. This is due to the past experience observation that the prosthesis has a tendency to migrate in the aortic direction during deployment. This phenomenon is likely a result of the stored torque in the delivery system when introduced from the femoral artery. For ease of prosthesis arch transit, the delivery platform is equipped with the Retroflex system from Edwards. Of note, the correct orientation of the prosthesis should be confirmed both visually prior to placement into the introducer sheath and angiographically prior to deployment. Next, transvalvular flow is severely depressed by rapid ventricular pacing. The valve is then balloon expanded rapidly and implanted in position. If there is moderate to severe aortic insufficiency from a paravalvular leak after the deployment, a second ballooning with a larger volume is performed under conditions of rapid ventricular pacing.
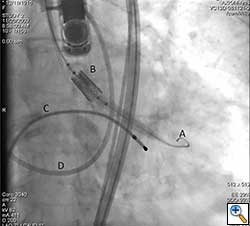
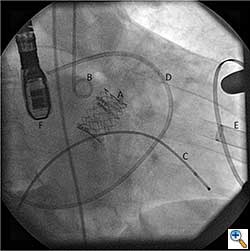
After valve implantation, it is critical to confirm valve competence, visualize coronary flow, and check for aortic injury by performing echocardiography and completion angiography (Figure 3). In the stabilized patient, the large femoral sheath is removed while maintaining wire access, ensuring no damage to the iliac artery. Once flow is confirmed in the proximal and distal femoral artery, the guidewire can be removed. The artery is clamped on either side and the arteriotomy is closed primarily with interrupted 5-0 or 6-0 prolene. The incision site is closed in standard fashion with absorbable suture in layers. The femoral arterial and venous sheaths in the contralateral groin may be left in place and then removed when the activated clotting time reverses to normal. Patient is taken back to the cardiothoracic intensive care unit for postoperative recovery.
Transapical aortic valve implantation
Similar to the transfemoral approach, the procedure is performed in a hybrid operating room involving a team of cardiovascular surgeons, interventional cardiologists, perfusionist, and cardiac anesthesiologists. The patient is positioned in a supine manner with slight elevation of the left chest, with rest of the preoperative set-up being similar to the described transfemoral approach.
Femoral venous access is obtained percutaneously and a transvenous pacing wire is advanced into the right ventricle over a 7 French sheath. Femoral artery access with a 6 French sheath is also achieved percutaneously and a diagnostic pigtail is advanced to the level of the aortic root. Angiography is then performed to determine optimal C-Arm position for valve deployment.
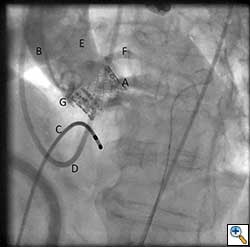
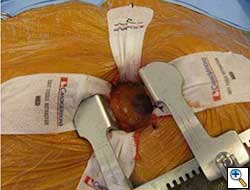
Once optimal C-Arm position has been determined, a small left anterior mini-thoracotomy is made to obtain access to the cardiac apex (Figure 4A, B). Two perpendicular 2-0 prolene pledgeted mattress sutures (or purse string depending on surgeon’s preference) are placed at the apex, taking care not to damage the left anterior descending artery. After the patient is heparinized, the apex is punctured and a guidewire is placed into the left ventricular cavity and advanced through the aortic valve into the aorta under fluoroscopic guidance. Upon confirming position, a 7 French sheath is advanced over the guidewire into the left ventricle and out into the aorta. Using a wire exchange technique under flouroscopy, a stiff guidewire (Lunderquist or Extra-stiff Amplatz) is positioned into the descending thoracic aorta with the aid of a JR4 guide catheter (Boston Scientific Corporation, Massachusetts).
Over the stiff guidewire, the apex opening is serially dilated to accommodate a 24 French sheath that is introduced up to the mid-ventricular cavity. It is critical to make sure that the mitral valve subannular apparatus is not involved during the apex dilatation and in the positioning of the valve deployment system. Flouroscopy and echocardiography can play an important role in confirming proper placement of the valve sheath system.
Once the positioning of the valve is confirmed by echocardiography and fluoroscopy, balloon valvuloplasty is performed in a similar fashion to the transfemoral approach described earlier. Upon restoring stable hemodynamics, the mounted Sapien valve on a balloon catheter is introduced transapically and proper position is confirmed by echocardiography and angiography (Figure 4C). For the transapical approach, the recommended position of the prosthesis is 50% of the prosthesis on the aortic side of the aortic annulus and 50% on the ventricular side of the annulus. Since the delivery system is much shorter compared to the transfemoral approach, the direct apical access to the aortic annulus results in less stored torque in the system. Therefore on deployment, the prosthesis does not have the tendency to migrate. During a period of rapid ventricular pacing, the valve is rapidly deployed by expanding the balloon (Figure 5). The valve is not re-ballooned unless there is moderate to severe paravalvular leak. The ventricular sheath is then removed and hemostasis is achieved by tying down the apical purse string sutures during rapid ventricular pacing. Great care must be taken in tying these sutures, as too much tension or torque may cause the ventricular apex to tear and cause uncontrollable bleeding.
Postoperative Management and Complications
Patient is taken to the cardiothoracic intensive care unit with central venous access and Swan Ganz catheter in place. Although mild aortic regurgitation is common and may not require major cardiac support, a patient with moderate to severe aortic insufficiency postoperatively may require prolonged inotrope support. Given these patients suffered from long term aortic stenosis, their postoperative course is similar to the patient undergoing open aortic valve replacement for aortic stenosis. Therefore, they may display labile hemodynamics, require inotropic support with volume loading to optimize cardiac index/ perfusion along with afterload reduction. It is critical to remember that even though these patients seemed to have undergone a minimally invasive procedure, their comorbidities along with a diseased heart from long-term aortic stenosis makes them a very sick cohort of patients.
Complications after successful valve implantation are primarily related to vascular access and thromboembolic phenomenon. Patients are at risk for myocardial infarction, stroke, and contrast induced nephropathy. New atrioventricular nodal block may also occur, though infrequent. Patients undergoing transapical aortic valve implantation may also be susceptible to new onset mitral regurgitation from inadvertent manipulation of the mitral subannular apparatus, pericardial effusion with ensuing tamponade, pneumothorax, and late apical rupture. Patients need to be monitored for complications related to the vascular access site, including groin seromas/ abscess, femoral artery dissection, thrombosis with risk for lower extremity ischemia, femoral artery pseudoaneurysm, and retroperitoneal hematoma.
Discussion
In 2002, Alain Cribier and associates described the first transcatheter aortic valve implantation in a human [4]. Since then the technique has undergone much refinement and modifications, and has emerged as a technique with great clinical significance. In the future, the trained cardiovascular surgeon with endovascular skills will be a unique physician with the versatility to perform the full gamut of treatment options for aortic stenosis: open versus transcatheter aortic valve implantation (via either the transfemoral or transapical approaches). Some experts believe that in the future up to 30% -40% of aortic valve intervention may be performed utilizing catheter-based techniques.
At present, only two types of transcatheter aortic valve systems are available in the United States as part of clinical trials – Medtronic CoreValve Revalving device (Medtronic Inc, Minneapolis, Minnesota), and the Edwards Sapien valve (Edwards Lifesciences Inc, Irvine, California). Both systems have been well studied in human trials and available in Europe as CE Mark approved products. The second generation Edwards Sapien platform (Sapien XT) has been introduced and it is only available in Canada and Europe at this time. The major modification includes reducing the profile of the femoral sheath system to 18 French. The important differences between the two are noted in Table 1.
Table 1: Differences between the Medtronic CoreValve and Edwards Sapien valve systems
| Medtronic CoreValve | Edwards Sapien valve | |
|---|---|---|
| Minimum femoral artery diameter required | 6.5 mm | 7 mm |
| Composition | Porcine pericardial with nitinol stent | Bovine pericardial with steel stent |
| Delivery system size required | 18 French | 18 French (Sapien XT only)* 22 French (23 mm valve) 24 French (26 mm valve) |
| Native annulus size feasible for implant | 19 mm to 27 mm | 17 mm to 25 mm |
| Mechanism of implantation | Self-expanding | Balloon expandable |
| Ventricular rhythm at time of implant | Beating heart | Rapid ventricular pacing |
* Currently not available in the U.S.
The selection of patients for transcatheter valve implantation is critical. Current trials primarily focus on those patients who are poor candidates for open repair and carry significant comorbidities as based on Society of Thoracic Surgeons score or EuroSCORE. These studies strongly suggest that transcatheter aortic valve implantation is a feasible and reliable treatment option in this patient population. Although patients may be candidates for either the transfemoral or the transapical approaches, the typical algorithm is to pursue the transapical method if the patient fails to be a candidate for the transfemoral approach, although there is no randomized data to support one method over the other. Future studies should shed light on this topic. Rather than see these two approaches as one versus the other, most likely, surgeons who are well trained in both techniques, treating them as complementary to one another would be able to offer the best treatment option for the patient.
Bleiziffer and associates reported on one of the largest series comparing the transapical versus transfemoral approaches [5]. This was a retrospective analysis of a cohort of 203 high risk patients who underwent transcatheter aortic valve implantation via a transapical (n=50) or transfemoral (n=153) approach. Of note, the transapical approach was only performed in those patients who failed to be candidates for the transfemoral approach. Both groups had similar 30 day and 6 month survival (88.8% and 80.1% for transfemoral; 91.7% and 73.4% for transapical). Both groups showed an improvement in the New York Heart Association class after transcatheter valve replacement. But the incidence of neurologic events was higher in the transfemoral group compared to the transapical group (7% versus 0%). The study showed that both techniques can be employed successfully at a single institution. The higher rate of neurologic events with the transfemoral group suggests that the algorithm of “transfemoral first-transapical second if no femoral access” may not be the ideal treatment pathway, and perhaps a more sophisticated patient screening process is needed. This study was also confounded by the fact that the center utilized both the Medtronic CoreValve and the Edwards Sapien valve, and assumed that this would have no difference in the outcomes. Future studies should elaborate on these issues.
References
- Safian RD, Berman AD, Diver DJ, McKay LL, Come P, Riley M, Warren S, Cunningham MJ, Wyman MR, Weinstein JS, Grossman W, McKay RG. Balloon aortic valvuloplasty in 170 consecutive patients. New England Journal of Medicine 1988; 319: 125-130.
- Bande M, Michev I, Sharp ASP, Chieffo A, Colombo A. Percutaneous transcatheter aortic valve implantation: past accomplishments, present achievements and applications, future perspectives. Cardiology in Review 2010; 18: 111-124.
- Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, Mueller R, Menichelli M, Schmidt T, Zickmann B, Iversen S, Stone GW. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 2006; 114: 1616-1624.
- Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106: 3006-3008.
- Bleiziffer S, Ruge H, Mazzitelli D, Hutter A, Opitz A, Bauernschmitt R, Lange R. Survival after transapical and transfemoral aortic valve implantation: talking about two different patient populations. Journal of Thoracic and Cardiovascular Surgery 2009; 138: 1073-1080.