Introduction
Primary tumors and congenital defects of the chest wall are a heterogeneous group of clinical conditions posing a common problem when it comes to reconstructing the defect [1]. While small defects, less than 5 cm anteriorly and 10 cm posteriorly, are not always repaired, resection of larger areas affects chest mechanics and breathing patterns, and can give rise to lung herniation or scapula entrapment.
A number of prosthetic materials, including GORE-TEX® and Marlex Mesh sandwiched with methylmethacrylate cement, have been described to restore the rigid chest wall contour [2-4]. We discuss the role of a new rigid system for chest-wall reconstruction, consisting of moldable titanium bars and rib clips (Strasbourg Thoracic Osteosyntheses System - STRATOS™; MedXpert GmbH, Heitersheim, Germany) (Figure 1) used in combination with a biological patch (Veritas® - Synovis Life Technologies, Inc.). This combination of technologies we believe has advantages over current techniques.
 |
| Figure 1: STRATOS™ implants |
The use of malleable metal bars to restore anatomic rib continuity is intuitively likely to preserve the mechanics of ventilation better than a soft patch repair. A semi-rigid patch prevents herniation and scapula entrapment but leaves the mechanics of unified chest wall movement irreversibly altered.
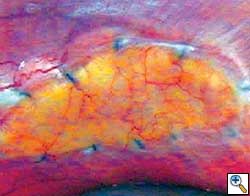
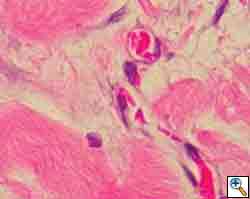
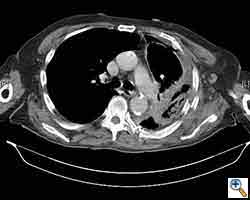
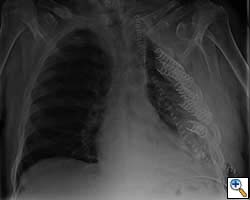
The use of a flexible patch sutured over the framework of titanium bars is often needed to provide a base for overlying soft tissue reconstruction, and to tissue prevent herniation between the bars. Large synthetic polymer patches are an infection risk. Veritas® pericardium is acellular, biocompatible with an extremely low level of extractable DNA, and has been used in closing large fascia defects in abdominal wall and breast surgical practices, particularly in the presence of established infection. It is clearly a new product for which there is so far limited clinical data, but the early experience at these sites suggests it provides good fascial support to surrounding autologous tissue and resists infections. The Veritas® Collagen Matrix fosters remodeling by supporting both angiogenesis and cellular ingrowth. (Figures 2,3) Angiogenesis within the implant establishes new capillaries, thereby making the implant environment hospitable. Fibroblasts within use the implant as a framework or “scaffold” for deposition of new collagen cells. The remodeling stage progresses within the patch with continued deposition of cells and reorganization of the new synthesized collagen and blood vessels. The result is the seamless integration of the implant into the surrounding tissue. Animal studies demonstrate that after as little as one month, the implant is histologically indistinguishable from the host's native tissue.
Patient selection
This technique is applicable to a wide range of clinical conditions in order to achieve good cosmetic and functional results.
Currently we employ this technique in the following situations:
- Chest wall resection for malignancy (Figures 4-5)
 |
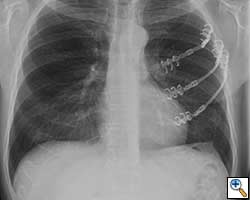 |
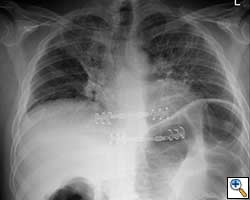
| Figure 4: Model showing application in chest wall resection. | Figure 5: Postoperative chest x-ray showing the implants |
- Functional repair of chest wall abnormalities (for example, pectus excavatum) (Figures 6-8)
- Fixation of massive chest wall injury (Figures 9-11)
Operative steps (for reconstruction after chest wall tumor removal)
- After the resection of the tumor en bloc with the ribs involved with clear sufficient margins, the reconstruction begins with the preparation of the STRATOS™ clips to follow the contour of the chest. Special tools are available for this purpose. (Figure 12)
 |
| Figure 12: Clip plying tool. |
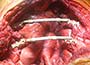
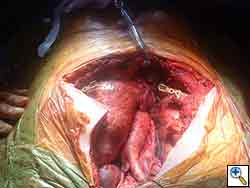
- Subsequently 2 titanium clips are crimped on each side of the defect. (Figure 13) by means of specifically designated tools. (Figure 14)
 |
 |
| Figure 13: After specimen resection the clips are applied to the free ends of the rib. | Figure 14: Clip crimping tool. |
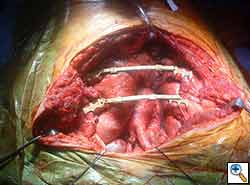
- Each rib defect is, subsequently, replaced with a titanium rib bridge. (Figure 15) The connecting bar is then cut to size using carbide-tipped pliers and is hand-contoured to the shape of the chest wall. Finally, the bar is crimped onto the clips. (Figure 16)
 |
 |
| Figure 15: The clips have been joined by the titanium bars. | Figure 16: Bar crimping tool. |
- Particular care must be taken to elevate the intercostal muscle and neurovascular bundle to avoid chronic neuropathic pain. Three different rib clips are available to suit the anatomical situation: 0°, 22.5° and 45°. A full set of instruments is provided to shape the clips and bars together with dedicated cutters. The technique does not need screws, cement, or glue.
- The Veritas® biological patch is laid over the implant (Figure 17) and sutured into place with a running PDS suture.
 |
| Figure 17: Veritas® patch laid on top of the implants. |
- Two chest drains are put in the pleural cavity and a drain is also placed at the end of the reconstruction. Plastic surgical reconstruction over this repair can then proceed.
- The pleural drains are maintained on suction until the first postoperative day and then placed on water seal if there is no air leak. They are removed on the first and third postoperative day. The soft tissue drain is removed in the fourth postoperative day.
Tips and pitfalls
- Making the incision too small when using this technique to repair asymmetrical pectus deformity leads, inevitably, to a malapposition of the clips with the resulting weakness of the repair.
- It is particularly useful to secure the anterior part of the clips with a clamp during the initial application to avoid misalignment.
- Fixation with stainless steel wires is not recommended as it weakens the ribs.
- During the initial crimping it is useful to press in the middle part of the clips rather than make full use of the notch in the instrument, this avoids rotation on the ribs.
- If the defect requires more than one bar we tend to use those with indentation all along their length, as these can be cut and used to bridge to ribs, rather than using two traditional bars with indentations only at the extremities.
- It is advisable to put a patch over the implant to avoid lung or soft tissue herniation anteriorly or laterally, or scapula entrapment posteriorly.
- Try to spare all the muscular layers to obtain the best cosmetic results in patients with chest wall trauma or defects. The same principle applies in patients with chest wall tumors even if, at times, parts of the muscles are removed en bloc with the lesion to achieve negative margin.
- A limitation of this technology is poor anchoring of the clips to either the transverse vertebral processes or anterior costal cartilages. The slimmer, cylindrical profile of the skeletal elements here does not allow a good fit with the currently available equipment.
Results
Many different methods have been proposed to stabilize the chest wall after resection. The rigid reconstruction of chest wall defects with GORE-TEX® felt or mesh-cement sandwiches has been widely reported. However, it does not restore normal chest wall mechanics and is vulnerable to catastrophic prosthetic infections. We believe that the application of these newer surgical materials is likely to provide important clinical benefits over established techniques. It is also an advantage that the same techniques can be applied to a number of clinical scenarios. We are particularly interested in their use in massive chest wall trauma, where early restoration of chest wall mechanical integrity may well play a part in reducing respiratory morbidity. We treated 9 patients (4 pectus corrections, 3 chest wall resections for malignancy, 1 flail chest fixation and 1 chronic pain syndrome from rib malunion) with excellent results.
Here we present, as a paradigm of repair, the case of a 65 year old gentleman referred to our institution for an indolent mass in the left anterior chest wall. Repairs for other conditions follow the same rules. This mass had been stable in size for more than 12 months and the patient was prompted to seek medical attention as, after weight loss due to dieting, he felt the mass was uncomfortable while sleeping. Apart from coronary stenting for angina, the past medical history was unremarkable.
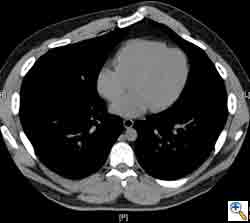
Computed tomography scan of the chest showed a mass protruding from the cortex of the third rib. A percutaneous core biopsy was non-diagnostic. We proceeded to an open incisional biopsy. This was reported as showing a spindle cell neoplasm. The patient was therefore scheduled, after multidisciplinary meeting discussion, for radical surgery.
At surgery, the mass was arising from the cortex of the third rib, and solid measuring 5x8 cm. There was no macroscopic involvement of the surrounding extra-thoracic musculature or of the underlying lung, the mass being confined to the rib and intercostal muscles. The patient underwent resection of the second to fourth ribs and the rhomboid muscle as well, in the attempt to get clear margin. The large antero-lateral defect was eventually reconstructed using 2 mouldable titanium bars. To prevent lung herniation through the defect, we reconstructed the layers anatomically using a biological patch (Veritas®). The final histopathology was a low grade sarcoma of the chest wall with clear resection margins.
He made an excellent recovery with daily physiotherapy to encourage shoulder movements. He was discharged with oral analgesia on postoperative day 4. At 1-month follow-up the patient had no pain at all, discontinued analgesia on postoperative day 17 and demonstrated a full range of shoulder movement. His chest x-ray was unremarkable and the wound healed without complication.
This technique is applicable to a wide range of surgical problems, easy to use and there is reason to believe it is superior to older approaches.
Disclosure
The Authors wish to disclose that they have no financial relationships with the companies manufacturing the products described in this presentation. The cost of the implants, patches and imaging have been supported by the United Kingdom National Health Service.
Acknowledgments
The Authors wish to thank the Manufacturers of the devices described for the kind permission to reproduce the images.
References
- Athanassiadi K, Kalavrouziotis G, Rondogianni D, Loutsidis A, Hatzimichalis A, Bellenis I. Primary chest wall tumors: early and long-term results of surgical treatment. Eur J Cardiothorac Surg 2001;19:589-93.
- Mansour KA, Thourani VH, Losken A, Reeves JG, Miller Jr JI, Carlson GW, Jones GE. Chest wall resection and reconstruction: a 25-year experience. Ann Thorac Surg 2002;73:1720-5.
- Weyant M, Bains M, Venkatraman E, Downey R, Park B, Flores R, Rizk N, Rusch V. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85.
- Chapelier A, Macchiarini P, Rietjens M, Lenot B, Margulis A, Petit JY, Dartevelle P. Chest wall reconstruction following resection of large primary malignant tumors. Eur J Cardiothorac Surg 1994;8:351-7.
- Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg 1996;98:804–10.