ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Robotic Endoscopic Transmitral Septal Myectomy and Concomitant Mitral Valve Repair for Hypertrophic Obstructive Cardiomyopathy
N. AlJamal Y, Balkhy HH. Robotic Endoscopic Transmitral Septal Myectomy and Concomitant Mitral Valve Repair for Hypertrophic Obstructive Cardiomyopathy. April 2025. doi:10.25373/ctsnet.28745702
In this new CTSNet President’s Series, Dr. Husam Balkhy, president of ISMICS, showcases cutting-edge, totally endoscopic cardiac procedures from the University of Chicago. Watch for more videos in this series coming soon.
Patient Presentation
The authors present a symptomatic 54-year-old woman with hypertrophic obstructive cardiomyopathy (HOCM) and severe mitral regurgitation (MR). A transesophageal echocardiogram (TTE) showed asymmetrical interventricular septal hypertrophy measuring 2.46 cm with severe mitral regurgitation due to intrinsic mitral disease (annulus dilatation and posterior leaflet prolapse) and systolic anterior motion (SAM), which contributed to the obstruction of the left ventricular outflow tract (LVOT) with mean and max gradients of 55 mmHg and 123 mmHg, respectively (Figure 1).
Figure 1(a)

Figure 1(b)

Figure 1. (a) Preoperative transesophageal echocardiogram shows a bulging septum (a, between the two white +Trans.) [b] Left ventricular outflow tract mean and peak gradients.
Patient Position and Port Placement
The patient was taken to the operating room (OR), where she was positioned supine with a roll under her right side. The right shoulder was deflected posteriorly, and the right arm was placed at the level of the bed (Figure 2a). The bedside assistant and the scrub nurse were located on the right of the patient, while the robotic system came in from the left side and was docked to right chest ports in a standard configuration for robotic mitral valve (MV repair). Five ports were placed:
1. A working port (8 mm) with an XX small Alexis (soft tissue retractor) was placed in the fourth intercostal space, medial to the anterior axillary line.
2. A camera port (8 mm) was placed medial to the working port in the fourth intercostal space.
3. Two additional ports (8 mm) were placed in the second and sixth intercostal spaces, aligned with the working port.
4. A port for a third arm (left atrial retractor) was placed medial to the midclavicular line in the fifth intercostal space (Figure 2b).
General endotracheal anesthesia with a single-lumen tube was initiated. The femoral artery and vein were accessed through a 2 cm incision in the right groin. The authors prefer to cannulate the femoral artery above the inguinal ligament, where the artery is larger to minimize the risk of limb ischemia by preserving collateral perfusion from its branches. After administering heparin and under transesophageal echocardiogram (TEE) guidance to ensure proper positioning of the cannulas and the guidewires, the catheter in the right internal jugular vein, originally placed by the anesthesia team, was replaced with a superior vena cava (SVC) cannula, and a long right femoral venous cannula was positioned with the tip just below the SVC, along with a standard right femoral arterial cannula.
Figure 2(a)

Figure 2(b)
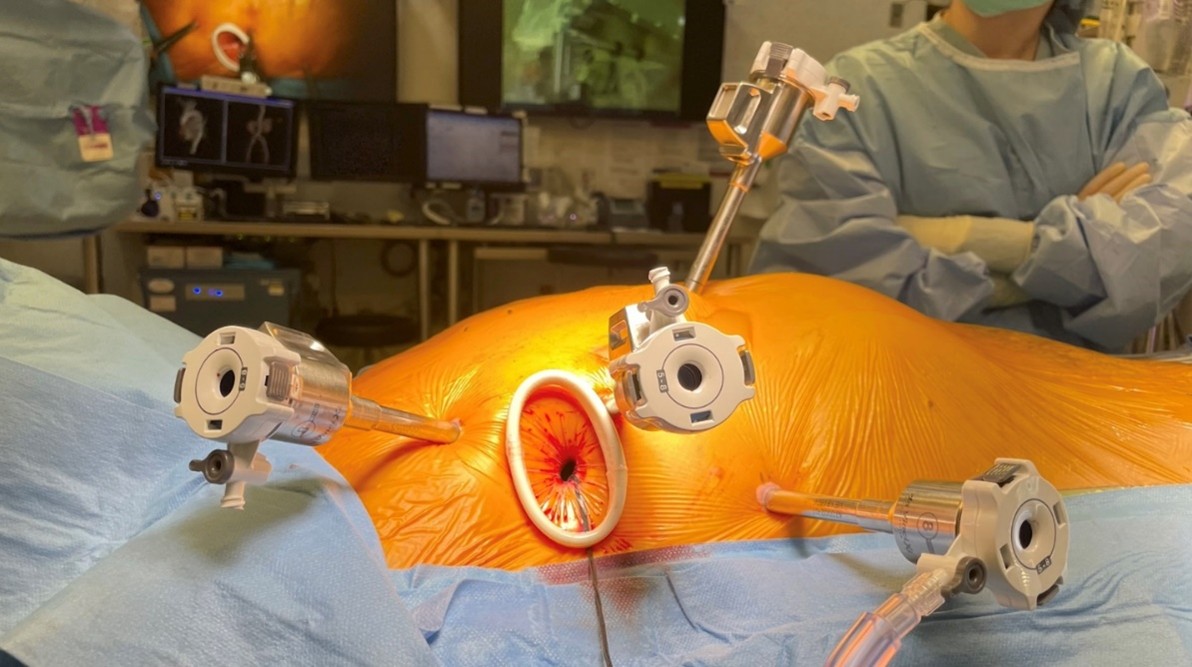
Figure 2. (a) Setup for a robotic interventricular septal resection. The patient was positioned with the right hemithorax elevated at a 30-degree angle, and the right arm was tucked by the patient’s side. (b) Robotic instrument arm trocar placement. The five-port technique includes: (1) an assistant port (12 mm) with an XX small Alexis retractor in the fourth intercostal space, placed medial to the anterior axillary line; (2) a camera port (8 mm) was placed medial to the working port for the camera; (3 and 4) two working ports (8 mm) were placed in the second and the sixth intercostal spaces, aligned with the assistant port; (5) a third (retractor) port was placed medial and one intercostal space below to the camera port.
Intrathoracic Setup
With an adequate activating clotting time, cardiopulmonary bypass was initiated, and the ventilator circuit was disconnected, after which the da Vinci Xi robotic system was docked. Under CO2 insufflation, a diaphragmatic retraction stitch using 3-0 Prolene was placed on the anterior medial, mid medial, and posterior medial of the central tendon of the diaphragm and brought out anterolaterally through the chest wall for retraction and improved exposure of the pericardium and right atrium. The pericardium was then opened longitudinally, anterior to the phrenic nerve. Two pericardial retraction sutures were placed and brought out posterior-laterally to the working port. The oblique sinus was opened, Sondergaard’s groove was developed, and the plane between the aorta and right pulmonary artery (PA) was developed. The procedure can be performed with either endoaortic balloon occlusion or a transthoracic Chitwood clamp. The authors’ standard approach for MV surgery is the endoaortic balloon; however, to fully decompress the aortic root and achieve a clearer view of the interventricular septum, the authors used the transthoracic Chitwood clamp with antegrade and retrograde cardioplegia. A retrograde cannula to the coronary sinus, placed through the right internal jugular, was placed by the anesthesia team with TEE guidance. The transthoracic Chitwood clamp was brought in through the second intercostal space in the midaxillary line. The aorta was cross-clamped in the mid-ascending region after dissection of the aortopulmonary window. A long percutaneous 14 G cardioplegia catheter was placed in the second intercostal space and positioned proximal to the cross-clamp. Antegrade del Nido crystalloid cardioplegia was administered in the root to achieve a flaccid diastolic arrest, and subsequent cardioplegia doses were given retrograde throughout the procedure.
Transmitral Myectomy
CO2 was used to flood the field at a flow rate of 2 L/min. A generous left atriotomy was made along Sondergaard’s groove, and the robotic dynamic atrial retractor was positioned to expose the mitral valve. With the aid of the robotic suction/irrigation system, the mitral valve was studied, and the leaflet coaptation was marked by measuring the long-axis length of the A2 and P2 segments. The mitral valve’s annulus was severely dilated with intrinsic posterior leaflet disease. The plan was to proceed with the myectomy portion and address the mitral valve pathology on the way out. A generous incision was made in the anterior leaflet from the medial trigone to the lateral trigone, leaving a 2 mm cuff of the anterior leaflet to the annulus to aid with the repair (Figure 3). The atrial retractor was then positioned to expose the interventricular septum through this incision.
Figure 3

Figure 3. (a) Measuring the distance from the medial to the lateral trigone to aid with closure planning. (b) A generous incision was made in the anterior leaflet from the medial trigone to the lateral trigone using Potts scissors, leaving a 2 mm cuff of the anterior leaflet adjacent to the annulus to aid with the repair. (c) Exposure of the interventricular septum and aortic valve through the mobilized mitral valve anterior leaflet.
The transmitral septal myectomy was performed by incising the thickened septum, extending from the 12 o’clock position counterclockwise to the 9 o’clock position (Figure 4a). The thickness of the incision was based on the preoperative TEE septum thickness. Muscle resection was extended to the midventricular level, opposite to the papillary muscle, to relieve any midventricular obstruction (Figure 4b and c). Using the high-definition 3D robotic camera visualization (either 30-degree or 0-degree angle), can facilitate identifying areas with incomplete myectomy and asymmetrical septum thickness. Thickened secondary chordae tendinea to the anterior leaflet were also visualized and transected when appropriate. Further evaluation of septum thickness and checking for ventricular septum defect (VSD) was achieved by filling the right ventricle, as coordinated by the perfusion team.
Figure 4(a)

Figure 4(b+c) [incorrectly labelled as ‘a’ and ‘b’ by author]

Figure 4. (a) Resection of septal muscle. The transmitral septal myectomy was performed by incising the thickened septum, extending from the right to the nadir of the right coronary cusp counterclockwise toward the anterior leaflet of the mitral valve. The thickness of the cut was based on the preoperative TEE septum thickness. (b) Myectomy extended to midventricular septum. (c) Trabeculation muscle opposite of the papillary muscles was excised.
Mitral Valve Repair and Completion of the Procedure
Upon completion of the myectomy, the anterior leaflet was reconstructed with a 0.8 mm wide ultra-thin bovine pericardium strip using running 4-0 Prolene sutures. Once completed, the mitral valve was then studied using a pressurized saline test, during which the leaflet coaptation level was marked and any leaks were noted. In this patient, two sets of neochordae (CV-4 PTFE) were placed on the posterior leaflet to position the coaptation level more posteriorly. Additionally, a mitral annuloplasty was performed with a 34 mm semirigid partial annuloplasty ring. It was sutured from trigone to trigone along the posterior annulus using a continuous running mattress with 3-0 Prolene sutures (Figure 5).
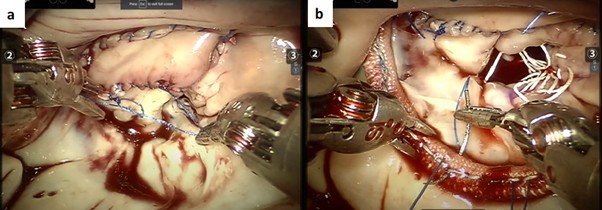
Figure 5. (a) Following completion of the myectomy, [a] the anterior leaflet was reconstructed with a 0.8 mm wide ultra-thin bovine pericardium strip using running 4-0 Prolene sutures. (b) Mitral valve repair was performed with two sets of neochordae and annuloplasty using a 34 mm semirigid partial annuloplasty ring. [b]: Transesophageal echocardiogram shows left ventricular outlet tract “pre- versus post-” septal myectomy mean gradients.
Once the mitral valve was felt to be competent on final saline testing, with no evidence of leakage and an adequately low leaflet coaptation level, the left atriotomy was closed with running 4-0 Prolene sutures. Once the heart was deaired, the angiocath was then removed, and the site was repaired with pledgeted 4-0 Prolene sutures, after which the cross-clamp was removed. Atrial and ventricular pacing wires were placed and brought out through the left atrial retractor port site. A 24 French channel drain was placed through the left atrial retractor port site and into the pericardial space through the oblique sinus. The pericardium was closed loosely with a V-Loc suture. The robot was undocked, and ventilation was resumed. Cardiopulmonary bypass was then discontinued, and both venous cannulas from the right internal jugular vein and right common femoral vein were removed. Protamine was administered, and the right common femoral artery cannula was then removed. Once hemostasis was confirmed, all port sites were closed.
Postoperative Transesophageal Echocardiogram
The postoperative transesophageal echocardiogram showed no evidence of MR or LVOT obstruction. The interventricular septum was thinner, and the mean gradient improved from 55 mmHg preoperatively to 5 mmHg postoperatively. The patient was discharged on the fourth postoperative day and was doing well, with complete resolution of symptoms at the three-month follow-up visit.
References
- Maron MS, Maron BJ. Hypertrophic cardiomyopathy - Authors' reply. Lancet. 2013;381(9876):1457-8.
- Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, et al. Management of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79(4):390-414.
- Morrow AG, Reitz BA, Epstein SE, Henry WL, Conkle DM, Itscoitz SB, et al. Operative treatment in hypertrophic subaortic stenosis. Techniques, and the results of pre and postoperative assessments in 83 patients. Circulation. 1975;52(1):88-102.
- Khalpey Z, Korovin L, Chitwood WR, Jr., Poston R. Robot-assisted septal myectomy for hypertrophic cardiomyopathy with left ventricular outflow tract obstruction. J Thorac Cardiovasc Surg. 2014;147(5):1708-9.
- Lillehei CW, Levy MJ. Transatrial Exposure for Correction of Subaortic Stenosis. JAMA. 1963;186:8-13.
- Korsik E, Meineri M, Zakhary WZA, Balga I, Jawad K, Ender J, et al. Persistent and acute postoperative pain after cardiac surgery with anterolateral thoracotomy or median sternotomy: A prospective observational study. J Clin Anesth. 2022;77:110577.
- Balkhy HH, Nisivaco S, Kitahara H, AbuTaleb A, Nathan S, Hamzat I. Robotic advanced hybrid coronary revascularization: Outcomes with two internal thoracic artery grafts and stents. JTCVS Tech. 2022;16:76-88.
- Jr. C. Idiopathic hypertrophic subaortic obstruction: robotic transatrial and transmitral ventricular septal resection. Operative Techniques in Thoracic and Cardiovascular Surgery. 2012;17:251-60.
Disclaimer
The information and views presented on CTSNet.org represent the views of the authors and contributors of the material and not of CTSNet. Please review our full disclaimer page here.




