ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Off-Pump Insertion of the Jarvik 2000 Left Ventricular Assist Device
Patient Selection
Left ventricular assist devices (LVAD) are now part of the routine armamentarium of surgeons for mechanical support of the failing heart. Despite improving perioperative and long-term results over the last decade [1-3], LVAD use is still associated with significant morbidity and mortality. Traditional left ventricular assist device (LVAD) implantation requires extensive dissection and use of cardiopulmonary bypass (CPB). Between 10% and 30% of patients suffer infection, bleeding, renal failure, respiratory failure, neurologic dysfunction, or right ventricular failure [4]. Many of these issues can be related to prolonged extracorporeal support in these very ill patients.
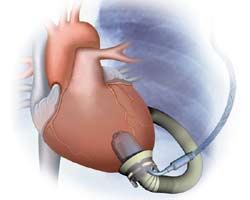
The Jarvik 2000 is an axial-flow pump involved in a FDA-approved phase II clinical pivotal trial in Status I patients as a bridge to transplantation (Figure 1). Although well over 100 implants have been performed in the United States and Europe during the feasibility trials, these devices have typically been inserted either by sternotomy or thoracotomy with the use of CPB. Our current approach is 2-pronged: in patients who are hemodynamically unstable leading to LVAD insertion, we have opted for placing these patients on CPB; for those who are hemodynamically stable, we perform the procedure off-CPB. To date, we have implanted this LVAD in 11 patients (2 who required CPB secondary to cardiovascular collapse). All patients were NYHA class IV on multiple inotropic drugs with impaired end-organ function. Implants without cardiopulmonary bypass had decreased transfusion requirements, fewer days on the ventilator, shorter ICU stays, and fewer adverse events including ventricular and supraventricular arrhythmias, need for dialysis, and bacterial and fungal infections [5]. Because of its size and construction of inflow and sewing ring, it is simple to place intrathoracically and is particularly suited for patients with previous heart operations in order to avoid redo-sternotomy.
Operative Steps
- After double lumen endotracheal anesthesia and insertion of transesophageal echocardiography probe, the patient is positioned in the lateral decubitus position with access available to the left femoral vessels. If significant concern for hemodynamic collapse exists, we will expose the femoral vessels for peripheral cannulation, otherwise, we simply place percutaneous 5Fr arterial and 7Fr venous sheaths. During our first few cases, we actually cannulated without going on full bypass. The presence of an arterial cannula can be reassuring and provide volume support.
- A generous thoracotomy through the 6th intercostals space is performed without sparing the serratus anterior muscle. Initially, this incision was carried far forward and included disarticulation of the costal cartilage so as to allow the elbow of the device to appropriately sit. Depending on the habitus of the patient, however, this fairly morbid extension can be avoided. If planning CPB, simultaneous dissection of the femoral vessels ensues.
- Tunneling for the driveline to the right upper quadrant is done prior to heparinization. This can be done at the insertion of the diaphragm or a counter-incision can be made through the intercostal space and then a second incision in the right upper quadrant. A 40Fr (if available) or 36Fr chest tube is passed through the tunnel with a long clamp. The driveline is much smaller than the pneumatic devices, but the connector to the controller for the Jarvik is slightly larger than the line. At the end of the case, we often will place a deep absorbable suture to close down the track, as well as a loose nonabsorbable suture around the drive line that is removed in 3 days.
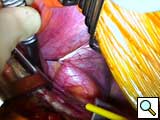
- To assist exposure, we often place a heavy silk suture in the central tendon of the left diaphragm and pull it caudad through a future chest tube site (Figure 2). After deflation of the lung, the inferior pulmonary ligament is divided up to the inferior pulmonary vein. The pericardium is opened anterior and parallel to, and at least 2 centimeters from the phrenic nerve.
- At this point, sizing of the outflow graft can be assessed so as to have gentle curve into the left costophrenic sulcus. We use a chest tube to assess the length realizing that the outflow graft will elongate when under systemic pressure. In general, the more cephalad the aortic anastomosis the better (potentially to assist with ascending stasis). Some have adopted a more aggressive strategy and have made a counter incision in the 3rd intercostal space to reach the underside of the arch and even the ascending aorta. The aorta is assessed for atherosclerotic disease. If large enough, we will not gain circumferential control of the aorta to avoid bleeding with the dissection and disruption of posterior plaque. Although some will proceed without systemic anticoagulation, we usually give 5-10,000 units of heparin.
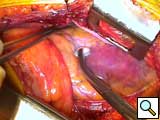
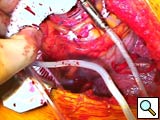
- After being tested on the back table, the device is brought on to the field. A finger from a sterile glove is tied on to the control connector that is subsequently inserted and firmly attached with heavy silk suture to the driveline chest tube. The driveline is subsequently pulled through the tunnel. Recently we have placed a 20mm ringed-gortex graft around the outflow graft to avoid graft kinking. This graft is secured nearly to the most proximal portion of the outflow with several 2-0 silk sutures. The Jarvik graft is cut to size with a bevel, a partial occluding vascular clamp is placed on the thoracic aorta, and the graft is sewn in place with a running 4-0 polypropylene suture (Figure 3). Felt buttresses or pledgets are used in more friable aorta or as deemed appropriate. A clamp is placed several centimeters above the anastomosis and hemostasis is assured.
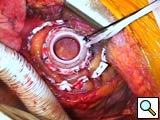
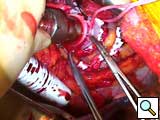
- The cardiac apex is positioned for placement of the inflow sewing ring. Even in the sickest patients, a small laparotomy pad can be placed infero-posteriorly to elevate the heart. The left anterior descending artery and apical insertion site are identified (Figure 4). In general, the sewing ring is positioned much like other LVADs. We opt for a slightly anterior apical position and attempt to have the device lay 1-2 cm away from and parallel to the ventricular septum. Teflon felt pledgets are cut to size and 2-0 braided polyester sutures are placed circumferentially. Depth of suture penetration is dependent in part on the thickness and sturdiness of the myocardium. In general, deep but not full thickness bites should be taken. These sutures are typically placed in quadrants with the sewing ring, aimed towards the aortic valve, being tied down after the first 2-3 sutures are placed. Great care should be used when tying these sutures, especially in patients with a recent myocardial infarction or dilated myopathies where tissue integrity could be quite weak. Typically, 12 sutures are required (with an occasional buttress suture as needed (Figure 5).
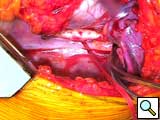
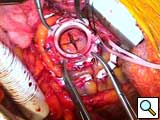
- The sewing ring’s insert has 4 “ears” which are controlled with small mosquito clamps. With an alert operative team and the patient in Trendelenburg position,a cruciate incision (Figure 6) is completed full thickness and the coring knife is inserted through the sewing ring, thus preventing any blood loss (Figure 7). In a continuous motion, the knife is removed, along with the plastic insert of the sewing ring (it has an attached silk tie that can be pulled together with the coring knife), and the Jarvik device is placed into the ventricule (Figure 8). Because of the smooth texture of the device, this maneuver is rapid, effortless, and completed within 1-2 beats of the heart.
- The outflow graft is deaired both proximally and distally to its clamp with a medium gauge needle through a pre-placed plegeted suture. Further air as well as alignment of the pump, especially in relation to the septum, is evaluated by echocardiography. When pleased with the position and de-airing, pumping is initiated at a low speed. Two umbilical tapes attached to the sewing ring are then tied to secure the pump in place.
- Inotropic and vasopressor support is used as needed to support the right ventricle. Hemodynamic and echocardiographic profiles at various pump speeds are observed. When adequate flow (both native heart and pump-assist) is obtained, protamine is used to reverse the heparin, drainage tubes are placed, and the chest is closed in standard fashion.
Postoperative Care
The postoperative management of these patients is similar to other circulatory assist devices.
- Risk of infection is quite low as the driveline and pump are small and there is little pump motion. Somewhat out of paranoia, we employ the Rematch-type recommended antibiotic protocol for 48 hours (Vancomycin 15 mg/kg i.v. 1 hour preoperatively, every 12 hours x 48 hours; rifampin 600 mg i.v. 1-2 hours preoperatively, daily x 48 hours; fluconzazole 400 mg i.v. preoperative and each day x 48 hours; Levofloxacin 500mg 1 hour preoperatively and each day x 48 hours; mupirocin 2% nasal ointment preoperatively and twice daily x 5 days).
- We have adopted a less aggressive initial anticoagulation policy than many centers. We wait at least 24 hours before initiating heparin and slowly raise their aPTT to 50-70 seconds prior to transitioning to warfarin (goal INR 2.5-3.5) and low-dose aspirin. We have had some delayed bleeding with aggressive anticoagulation. A concern with placing the outflow graft onto the descending aorta is the potential to have static blood flow in the aortic root especially if the aortic valve is persistently immobile. Initial recommendations were to turn the pump to a low speed (level 1) to allow for ventricular filling and ejection, thereby opening the aortic valve and “washing” it from stagnant blood flow. We are comforted with the newer Intermittent Low Speed controller that automatically turns the flow down on the pump to allow this same function occur, theoretically decreasing thromboembolic events.
- Hemodynamically, we maintain inotropic drugs for right ventricular support as long as necessary. If necessary, we will transfer patients from intensive care on low dose milrinone or dobutamine while they initiate more aggressive physical therapy. The pump is very afterload dependent. Usually, prior to discharge, we will re-initiate angiotensin converting enzyme inhibition or beta-adrenergic blockade to maintain mean arterial pressures in the 70-80 mmHg range.
Explant at time of transplant
An advantage to this approach for first time surgery patients is the relative ease of reoperation at the time of transplantation (no redo-sternotomy). That said, control of the outflow graft can be challenging. Ultimately, after initiating cardiopulmonary bypass, one has to clamp across the outflow graft to excise the heart. We have tried several different methods at the time of implant to make access of this region easier, but often the lung is nestled closely. The ringed gortex support graft can be used to peel-away the underlying outflow graft so as to not excessively injure the lung. The pump can be used at low speeds during this time to avoid retrograde flow and distention of the heart as you explant (functions as a vent) or a separate catheter can be placed in the right superior pulmonary vein with the pump turned off to accomplish the same end. We typically use an endoscopic stapling device with suture support to exclude the outflow to the aorta.
Comments
Our current algorithm is to use CPB in patients who are in extremis and cardiogenic shock. These patients likely benefit from the period of resuscitation provided by CPB during LVAD insertion [6]. However, in the larger group of very ill, but stable, class IV patients who need LVAD support, we attempt to place the Jarvik device without CPB. Is cardiopulmonary bypass bad? Both experimental and clinical evidence have demonstrated that CPB is associated with activation of a host of inflammatory mediators, priming of neutrophils, and increased lung edema. This issue has been widely debated with regard to coronary artery bypass surgery without clear resolution. However, patients requiring LVAD support are different than the standard CABG patient. Every organ system is often perilously close to failure, if not already there. Conceptually, decreasing the inflammatory insult on the lungs and kidneys will allow for better right ventricular and renal function, respectively. This benefit has been witnessed in high-risk patients with aortic stenosis undergoing placement of apical-aortic conduits. Conversely, the longer ICU stays and increased adverse events witnessed in our series of patients requiring CPB are quite likely not related to the use of CPB, but rather the premorbid condition of those particular patients.
Several other reports have described placement of continuous flow devices without cardiopulmonary bypass. Frazier described a patient in whom he placed this pump while briefly fibrillating the heart [7]. While relatively straightforward, this approach is limited in situations where it might be hard to defibrillate, such as patients with previous cardiac surgery or those with sick, distended hearts. This same group has also reported placement of the Jarvik 2000 with an anterior, intraperitoneal approach without bypass [8]. Although this technique is attractive in reoperative situations, exposure may be challenging.
Tips & Pitfalls
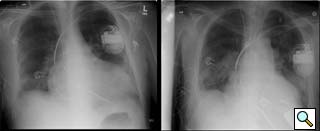
Figure 9: Pre- and postoperative chest radiographs in a 6’7” male demonstrating ventricular decompression.
- This technique should not be used in patients in extremis or cardiogenic shock. These patients likely benefit from the period of resuscitation provided by CPB during LVAD insertion. Other classic indications for performing this procedure on CPB include suspected ventricular thrombus or concomitant procedures (i.e. repair or replacement of an incompetent aortic valve).
- As with any patient requiring mechanical circulatory support, protection and function of the right ventricle is crucial. This point is accentuated when in the thoracotomy position as there is no access to the right side. As such, patient selection is very important. Several risk factors for biventricular support have been identified including females, dilated cardiomyopathies, and central venous pressures greater than 20 mmHg. Clearly, all measures to maximize right ventricular performance should be incorporated in the operative strategy, including minimizing transfusions, avoiding hypoxia and acidosis, prudent use of inotropic drugs, and liberal use of nitric oxide. That said, the off-pump approach is attractive, as avoidance of extracorporeal circulation and decreased transfusion requirements may lessen the risk of exacerbating end-organ function.
- Some will argue that the output of continuous flow pumps like the Jarvik 2000 is inadequate for certain patients. Although intrathoracic placement of the Heartmate pump has been described [9], pump size limits this approach to larger patients. The Jarvik 2000 has provided reliable and sufficient outputs for all of our patients (Figure 9 is a pre and postoperative CXR showing LV decompression in a patient that was 6’7” with a body surface area of nearly 2.3). That said, the Jarvik 2000 is an assist, and not replacement device.
- We have found that one should delay the urge to restore pulsatile flow (i.e. turn down the pump speed) until the patient has been off inotropic drugs for several days. The concern for thrombus generation on a closed aortic valve is real. However, the advent of the Intermittent Low Speed controller (turns speed to level one automatically every minute for several seconds) allows washing of the valve. Early attempts to turn the pump down can result in low cardiac output syndrome, pulmonary congestion, and right heart dysfunction.
References
- Long JW, Kfoury AG, Slaughter MS, et al. Long-term destination therapy with the HeartMate XVE left ventricular assist device: improved outcomes since the REMATCH study. Congest Heart Fail 2005;11:133-8.
- Park SJ, Tector A, Piccioni W, et al. Left ventricular assist devices as destination therapy: a new look at survival. J Thorac Cardiovasc Surg 2005;129:9-17.
- Pagani FD, Long JW, Dembitsky WP, Joyce LD, Miller LW. Improved mechanical reliability of the HeartMate XVE left ventricular assist system. Ann Thorac Surg 2006;82:1413-8.
- Deng MC, Edwards LB, Hertz MI, et al. Mechanical circulatory support device database of the International Society for Heart and Lung Transplantation: third annual report--2005. J Heart Lung Transplant 2005;24:1182-7.
- Selzman CH, Sheridan BC. Off-pump insertion of continuous flow left ventricular assist devices. J Card Surg 2007;22:320-2.
- Selzman CH, Chang PP, Vernon-Platt T, et al. Use of the Jarvik 2000 continuous flow left ventricular assist device for acute myocardial infarction and cardiogenic shock. J Heart Lung Transplant 2007;26:756-8.
- Frazier OH. Implantation of the Jarvik 2000 left ventricular assist device without the use of cardiopulmonary bypass. Ann Thorac Surg 2003;75:1028-30.
- Frazier OH, Gregoric ID and Cohn WE. Initial experience with non-thoracic, extraperitoneal, off-pump insertion of the Jarvik 2000 Heart in patients with previous median sternotomy. J Heart Lung Transplant 2006;25:499-503.
- Gregoric I, Wadia Y, Radovancevic B, et al. HeartMate vented-electric left ventricular assist system: technique for intrathoracic or intraperitoneal implantation via a left thoracotomy. J Heart Lung Transplant 2004;23:759-62.