ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Bilateral VATS Pulmonary Vein Isolation, Left Atrial Appendage Excision, Directed Partial Cardiac Denervation and EP Mapping (MiniMAZE-Wolf Technique)
Patients are selected for bilateral closed chest pulmonary vein (PV) isolation, left atrial appendage excision, directed partial cardiac denervation, and PV electrocardiograms (MiniMAZE-Wolf technique), based on the following criteria...
Patient Selection
Patients are selected for bilateral closed chest pulmonary vein (PV) isolation, left atrial appendage excision, directed partial cardiac denervation, and PV electrocardiograms (MiniMAZE-Wolf technique), based on the following criteria: drug refractory atrial fibrillation (AF), inability to tolerate or unwillingness to take anti-arrhythmic or anti-coagulation therapy, and no significant valvular or obstructive coronary artery disease. The suitability for operation and one lung anesthesia should also be determined. Patients undergo preoperative in home electrocardiographic monitoring to define frequency and duration of atrial fibrillation (CardioNet, San Diego, CA). Initially, only patients with paroxysmal AF were evaluated for operation. However, several candidates were found to have chronic AF, including several with complications from anticoagulation therapy. Currently, patients with paroxysmal and some selected chronic AF are candidates for the Wolf miniMAZE. A preoperative 64 slice CT scan is performed a few days before the procedure to delineate the pulmonary venous anatomy, assess the coronary tree, and to rule out thrombus in the LAA [1]. Approximately 30% of patients have had previous catheter-based pulmonary vein ablation, including some patients with as many as three previous unsuccessful catheter-based procedures.
Operative Steps
A bilateral video assisted thoracoscopic surgical (VATS) procedure was developed utilizing two 10mm ports and a 6cm non-rib spreading access port. A central line and peripheral arterial line are employed. A double lumen endotracheal tube is placed and position confirmed with bronchoscopy. We have switched from intraoperative TEE to preoperative 64 slice CT scan to rule out LAA thrombus, as CT appears to be more accurate and provides additional information regarding pulmonary vein anatomy.
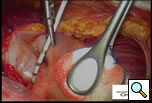
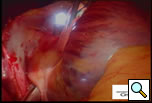
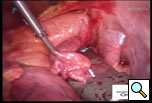
The patient is positioned with the right side up and is prepped and draped so as to have access to the lateral chest and the midline. A 10mm 30 degree thoracoscope is introduced into the right chest in the 6th or 7th interspace in the anterior axillary line at the level of the xiphoid (Right Side MiniMAZE), and the pericardium and pulmonary hilum are examined. A 6cm non-rib spreading access incision is made in the 3rd intercostal space and a soft tissue retractor inserted (Cardiovations, Sommerville, NJ). The overlying pericardial fat may be excised as necessary with appropriate attention to the phrenic nerve. The pericardium is opened anteriorly and parallel to the right phrenic nerve. Once the pericardium is opened, stay sutures are placed to maximize exposure (Figure 1). High frequency stimulation is then performed to identify and map vagal ganglionic plexi around the PVs and Waterson’s groove [2]. Pulmonary vein electrocardiograms (ECG) and corresponding surface ECGs are documented (Figure 2). Patients in sinus rhythm are then paced by placing the electrode on the pulmonary veins. Following EP mapping, blunt dissection is performed to open the oblique sinus between the right inferior pulmonary vein and the inferior vena cava.
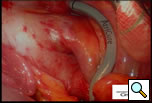
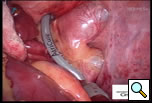
Additional blunt dissection is performed between the right superior pulmonary vein and the right pulmonary artery, lateral to the superior vena cava, to gently separate these structures. Through a separate port site a special articulating lighted dissector (Wolf dissector, Atricure Inc., West Chester, OH) developed for this procedure is placed under the inferior pulmonary vein and articulated to emerge above the superior pulmonary vein but below the pulmonary artery and lateral to the superior vena cava (Figure 3 and Video 1). The lighted dissector enhances the visual feedback of the operator, and the light generally transilluminates the adipose tissue, but not vascular structures, helping the surgeon guide the dissector in the proper plane between the pulmonary artery and vein. Once the dissector is around the right pulmonary veins a red rubber catheter, already attached to the dissector, is advanced, pulling the catheter behind the veins. The lighted dissector is then removed, and saved for use on the left side. The inferior jaw of a non-irrigated bipolar radiofrequency clamp (Isolator™, Atricure Inc., West Chester, OH) which is pre-attached to the other end of the rubber catheter, is directed around the pulmonary veins using the catheter as a guide. Once around the veins, the clamp is positioned on the left atrial cuff (Figure 4). Radiofrequency energy is delivered between two gold-plated electrodes embedded in the jaws of the clamp to create linear, transmural ablation lesions. During the isolation, objective feedback of transmurality is provided to the surgeon by graph and audible signal. Lesions can also be made along Waterson’s Groove, across the dome of the left atrium and down to the anterior mitral annulus with the Isolator clamp and/or an Atricure bipolar pen. A digital graph, located on the front panel of the generator, displays the conductance of the tissue clamped between the device jaws, and an audible signal indicates when the lesion is complete (usually about 8 seconds). Once isolation is complete, PV electrocardiograms are again obtained and pacing and high frequency stimulation are repeated to confirm bidirectional block. A bipolar atrial pacing wire is placed inside the pericardium; the wire and a chest tube are brought out through a port site. The incisions are closed and the patient turned to place the left side up.
Video 1
The left chest is then prepped and draped and similar incisions are made, although slightly more posterior (Left Side MiniMAZE). The pericardium is opened posterior to the phrenic nerve, and pericardial stay sutures are placed. After similar mapping of ganglion plexi and PV ECGs, the ligament of Marshall between the left pulmonary artery and the left superior pulmonary vein is divided using cautery (Figure 5 and Video 2). Through a separate port site, the special lighted articulating dissector is placed under the left inferior pulmonary vein and articulated to emerge above the left superior pulmonary vein. The red rubber catheter attached to the dissector is again withdrawn behind the veins. The bipolar radiofrequency clamp is then brought around the left pulmonary veins using the rubber catheter as a guide. The clamp is again positioned on the atrial cuff (Figure 6) and isolation lines are created. Once isolation is complete, PV ECGs are obtained and pacing and high frequency stimulation are repeated to confirm bidirectional block before withdrawing the clamp. Next an Ethicon EZ 45mm thick tissue stapler (green load) (Ethicon Endosurgery, Cincinnati, OH) is introduced through the port. The anvil of the stapler is placed against the left atrium and the left atrial appendage is gently positioned in the jaws (Figure 7). The tip of the stapler usually rests under the left pulmonary artery to include the entire atrial appendage. The stapler is then fired, and the left atrial appendage removed. Bipolar cautery is used to control occasional oozing from the cut atrial edges. A bipolar ventricular pacing wire is then secured inside the pericardium overlying the left ventricle and the pericardium is closed with interrupted sutures. The pacing wire and chest tube are brought out through the port site and the incisions are closed.
Video 2
Post-Operative Management
Post-operative discomfort has been managed very satisfactorily using intercostal nerve blocks and continuous in-wound infusions of local anesthetic (ON-Q Painbuster, I-Flow Corp, Lake Forest, CA). Patients who are not in sinus rhythm at the conclusion of the procedure undergo synchronized cardioversion while still under general anesthesia. Chest drains are placed to water seal and managed in the usual fashion. Once discharged, preoperative anti-arrhythmic medications are weaned and/or discontinued over the course of 12 weeks. All patients undergo postoperative in home electrocardiographic monitoring (CardioNet, San Diego, CA). If the patient has no AF on post-operative home monitoring, consideration is given for discontinuation of anticoagulation.
Disclosure Statement
I, Randall K. Wolf, M.D., serve as consultant and have a royalty agreement with AtriCure, Inc. I have stock and stock options in AtriCure, Inc.
I, E. William Schneeberger, M.D., serve as a consultant, do basic research, and perform proctoring of the mini MAZE procedure for Atricure. These activities are performed under the auspices of UC Surgeons, through the Division of Cardiac Surgery.
References
- Meyer CA, Wolf RK, Vagal AS, Mehall JR, Schneeberger EW, Strunk RS
“ECG-gated 64 Slice MDCT in the preoperative evaluation of patients prior to minimally invasive surgery for atrial fibrillation” J of Radiology, accepted for publication, abstract presented at Radiology Society of North America, 2006. - Mehall JR, Schneeberger EW, Schmerler DW, Kohut R, Wolf RK. “Technique of Identification and Isolation of Autonomic Ganglionic Plexi by Intraoperative Epicardial Electrophysiologic Mapping During Minimally Invasive Surgery for Lone Atrial Fibrillation” Ann Thor Surg, in press, abstract presented at Society of Thoracic Surgeons, 2006.
- Mehall JR, Kohut RM, Schneeberger EW, Merril WH, Wolf RK. "Absence of Correlation Between Symptoms and Rhythm in 'Symptomatic' Atrial Fibrillation Ann Thorac Surg, in review, abstract presented at Southern Thoracic Surgery Assocatiation meeting, 2006.