ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Composite Graft Replacement of the Aortic Root, Ascending Aorta, and Proximal Aortic Arch
Introduction and General Strategy
There are several indications to repair the aortic root and the ascending aorta. The most frequent one is the aneurysmal dilation of the aortic root ± ascending aorta that is associated with aortic valve dysfunction (in general regurgitation). In this case, the aortic valve leaflets may be fundamentally normal, but there is a loss of the sinotubular junction because of the dilation of the aorta. This may lead to insufficient coaptation of the leaflets and therefore cause valve-regurgitation. In other patients, the aortic valve leaflets may be abnormal because of bicuspid morphology (true bicuspid or pseudo-bicuspid valve) and the disease is associated with an enlarged ascending aorta. When the primary indication to operate on the patient is the aortic valve disease or myocardial revascularization, the ascending aorta is usually replaced very liberally (this means if the diameter is larger than 4.5 cm). Another situation that calls for the repair of the aortic root and ascending aorta is the patient that presents with severe aortic valve stenosis (either bicuspid or tricuspid aortic valve) and with post-stenotic dilation of the ascending aorta. In this category of patients, if the sinotubular junction is maintained and the aortic root diameter at the level of sinuses of Valsalva does not exceed 4 cm, the author usually proceeds with a separate aortic valve replacement and supracoronary replacement of the ascending aorta with a prosthetic graft.
During the last decade, the author has been increasingly aggressive in the replacement of the complete ascending aorta and has used a short period of mild hypothermic arrest very liberally to perform the distal anastomosis without aortic cross clamp. The main advantages of this strategy are: a most complete repair of the ascending aorta and an easier completion of the distal anastomosis at the level of the proximal aortic arch anastomosis. The experience of the author’s institution with moderate hypothermic circulatory arrest (core temperature 28-30°C and tympanic temperature 22-24°C) has been very encouraging, with a rate of neurological complications below 2% for elective surgery.
Preoperative assessment of patients requiring aortic root and ascending ± proximal aortic arch surgery generally includes a transthoracic echocardiography, and either an angio CT-scan or a magnetic resonance imaging to obtain all necessary information on the entire thoracic aorta. Proper description of the aortic dilation is necessary to optimally plan the procedure, especially the level of the distal anastomosis. In patients younger than 40 years and without any calcification of the coronary arteries in the cardiac CT-scan, angiography is not performed. However, this investigation is performed in every patient with significant cardiovascular risk factors and in those aged older than 40 years.
Clinical Vignettes
In the first video, the technique of composite-graft replacement of the aortic root is demonstrated (Video 1). The patient was 45-years-old and suffered from annulo-aortic dilation with severe aortic valve regurgitation. The annulus was 29 mm in diameter and there were numerous fenestrations at the level of the three commissures. The author felt that a David procedure was indicated, but the patient insisted on receiving the most definitive option, namely a mechanical valve. Resection of the aortic root and the aortic valve was performed and coronary orifices excised with a small rim of aortic tissue. A 27 mm composite graft was sized and fixation at the level of the aortic annulus was performed using interrupted Teflon-armed Ethibond 2.0 sutures. The stitches were done from the ventricular side. Alternatively the stitches can be done from outside in, leaving the pledgets outside of the aorta (this is particularly helpful to narrow a little bit of the aortic annulus). The coronary arteries were re-implanted with continuous 6.0 Prolene sutures, starting with the left ostium. To control hemostasis, cardioplegia is instilled into the aortic graft and fibrin sealant (Evicel, Ethicon, (New Brunswick, NJ, USA)) can be used. Finally the distal anastomosis is performed, the cross-clamp is removed, and careful de-airing is done through needle puncture of the prosthetic graft and CO2 insufflation in the operative field.
The second video illustrates the technique of a separate aortic valve and supracoronary graft replacement in a 68-year-old man, who presented with severe aortic stenosis combined with moderate regurgitation and post-stenotic dilatation of the ascending aorta (Video 2). The dimensions of the aortic root were practically normal with a diameter of 3.8 cm at the level of the sinuses of Valsalva and the sinotubular junction was well preserved. At this level, the quality of the aortic wall was excellent. Selective cardioplegia was administered into the coronary arteries. Aortic valve replacement was performed using a biological Edwards Lifesciences (Irvine, California, USA) Perimount Magna Ease 25 mm prosthesis and supracoronary graft replacement was performed with a Vascutek Terumo Anteflow (Renfrewshire, Scotland, UK) prosthesis 28 mm (sidearm 10 mm). The distal anastomosis was performed first, then the graft was cut to the appropriate length and the proximal anastomosis was performed last. No reinforcement material was used for the anastomoses. Total cross-clamp time was 52 minutes, including a mild hypothermic circulatory arrest of eight minutes and antegrade cerebral perfusion of five minutes. The patient recovered well and was discharged on postoperative day six.
Operative Technique
Cardiopulmonary Bypass and Myocardial Protection
Patients with aortic root disease and those with ascending aortic aneurysm are operated on through a median sternotomy. In the majority of patients suffering from arteriosclerotic aneurysm, the entire ascending aorta is usually dilated. If the arterial return of cardiopulmonary bypass is performed through the aortic cannulation, the cannula is placed into the most cranial part of the ascending aorta. An alternative to direct aortic cannulation, right subclavian artery cannulation is performed in all patients with type A acute aortic dissection and in those patients with expected difficult sternal reentry because of redo-procedure. Femoral artery cannulation is performed only exceptionally, for instance in patients who need emergency cannulation during mechanical regurgitation. Venous drainage is performed through right atrial cannulation using a two-stage cannula. Cardiopulmonary bypass is instituted in moderate hypothermia (either 32°C for simpler procedures or 28-30°C for those in which a short circulatory arrest is planned. A vent for the left heart cavity is placed through the right superior pulmonary vein and then through the mitral valve into the left ventricle in patients with significant aortic insufficiency. It is very important to avoid left ventricular dilation in case of ventricular fibrillation during the cooling period. Sometimes cross-clamping of the aorta is immediately necessary after the heart starts to fibrillate. Once the aorta has been cross-clamped, the author opens the ascending aorta and administers antegrade cold blood cardioplegia directly into the coronary orifices. In patients without aortic regurgitation, cardioplegia is administered through an aortic root cannula. Cold blood cardioplegia is repeated at 20-30 minutes intervals throughout the case.
Aortic Root Replacement
The specific techniques for aortic root repair - e.g., a David procedure or a composite graft replacement (Video 1) - are tailored to the pathology and based on the condition of the aortic valve, the sinuses of Valsalva, and the location of the coronary orifices. In fact, several operative techniques exist to repair the aortic root and the indication is handled individually for every patient. If the aortic sinuses are of normal size and the aortic valve leaflets are diseased, aortic valve replacement combined with supracoronary graft replacement is our preferred approach (Video 2). In some patients with normal aortic valve leaflets, as well as normal aortic sinuses, lone replacement of the ascending aorta using an undersized supracoronary graft is the preferred technique because it is usually effective enough to treat coexisting mild to moderate valve regurgitation by narrowing the sinotubular junction.
If the diameter of the aortic sinuses exceeds 4 cm (in patients younger than 70 years) and the aortic valve leaflets are diseased, aortic root replacement using the modified Bentall technique is indicated. In these patients, the coronary arteries are usually displaced cranially from the aortic annulus. Before starting with the reconstruction, the author usually completely resects the ascending aorta and the aortic root. The coronary orifices are excised with a small ring of aortic tissue and mobilized to allow tension-free re-implantation into the aortic prosthesis. The inclusion technique has not been used in the past 20 years, as the author believes that this technique is more frequently associated with pseudo-aneurysms of the coronary orifices in the mid- to long-term.
The procedure starts with the proximal suture of the composite graft prosthesis to the aortic annulus using separated mattress suture of 2.0 Ethibond backed with small Teflon pledgets. The first suture is placed at the level of the commissure between the left and right coronary sinuses and the following are stitched in a clockwise fashion (Image 1). The sutures are placed through the annulus with the pledgets left either on the aortic side thus everting the annulus, but in small annular diameters. The author prefers to stich these sutures from the ventricular side to the aorta, therefore the pledgets lie under the aortic annulus. Then the sutures are placed through the sewing ring of the composite graft prosthesis (Image 2) which is later on parachuted down to the aortic annulus (Image 3). The sutures are then tightened and the valve is placed into the outflow tract of the left ventricle (this provides excellent hemostasis).
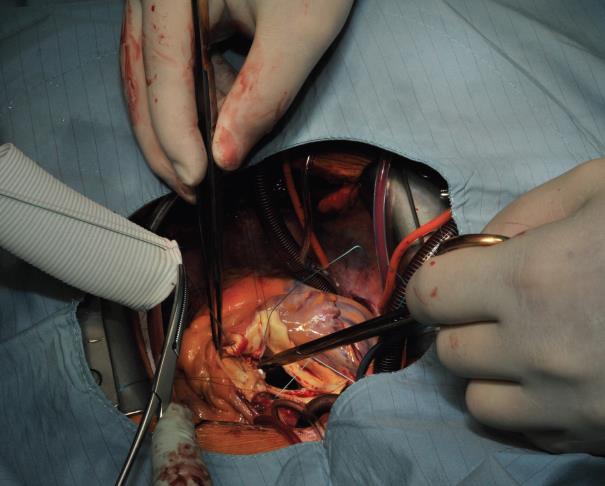
Image 1: Suturing the composite graft to the aortic annulus starts at the level of the commissure between the left and right coronary sinuses and is continued clockwise in back-end fashion up to the middle of the non-coronary sinus. Thereafter fore-hand technique is applied for the rest of the non-coronary sinus and the left coronary sinus.
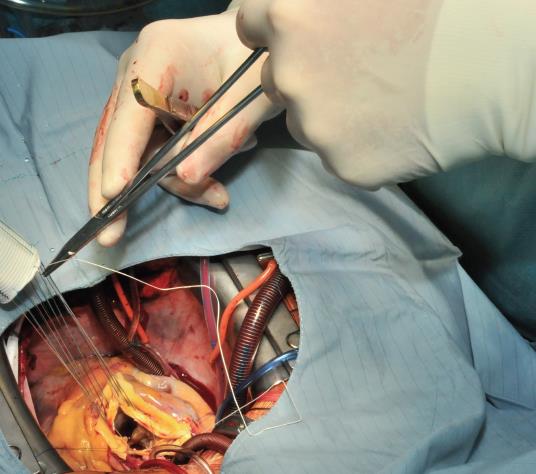
Image 2: The sutures are passed through the sewing cuff of the valve. In this case, the Teflon pledgets were placed below the aortic annulus. Alternatively, the stitches can be performed from the aortic side (outside-in) leaving the Teflon pledgets outside of the blood flow. This is especially favorable in case when the size of the aortic annulus has to be reduced.
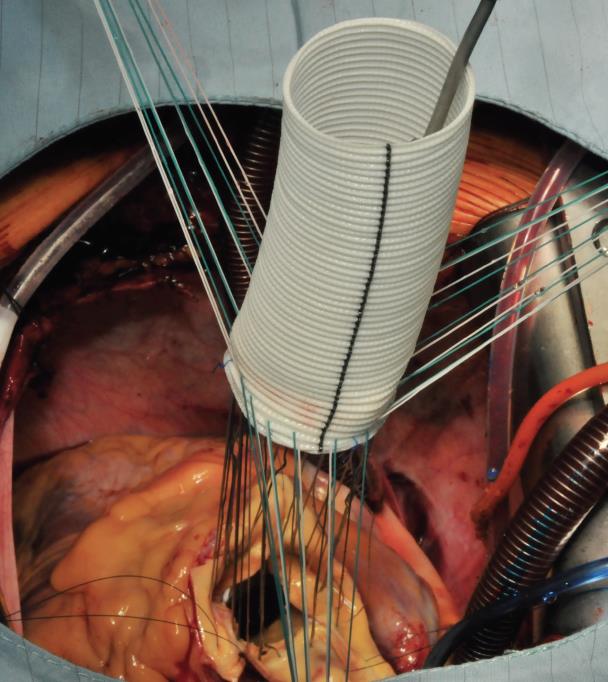
Image 3: The composite graft is then parachuted into the aortic annulus (mechanical valve) or placed in a supra-annular position when a bioprosthetic valve has been used. The sutures are tightened and cut. At this time, the core temperature has usually be brought to 28 or 30°C to proceed with the distal anastomosis at the level of the proximal (open) aortic arch.
Once the annular anastomosis has been performed, the temperature of the patient has usually reached the targeted core temperature of 28 to 30°C. The patient is placed in Trendelenburg position and Pentothal is administered. After the bispectral index has been reduced to zero, the extracorporeal circulation is interrupted. The arterial cannula is pulled out of the ascending aorta. At this time, the cranial part of the ascending aorta is resected and then selective perfusion catheters are placed into both common carotid arteries for antegrade selective cerebral protection. Usually it takes about 30 seconds to 1 minute to start cerebral perfusion. This technique of cerebral perfusion requires a constant flow of about 500 milliliters per minute, with a perfusion pressure not exceeding 60 mmHg. During hypothermic circulatory arrest with cerebral perfusion, the distal anastomosis is constructed at the level of the innominate artery. Usually a Vascutek Anteflow prosthetic graft is used which facilitates recannulation of the patient through the sidearm of the prosthesis, as soon as the distal anastomosis has been performed. Just before re-starting the extracorporeal circulation, the author pulls back the perfusion catheters and looks at the almost completed de-airing of the supra-aortic branches, the aortic arch, and the prosthesis that is clamped just below the sidearm. Control of the hemostasis can be done very easily at this stage around the whole anastomosis.
Thereafter, the work is continued at the aortic root. The anastomoses of the coronary orifices with the vascular graft are then performed. First a neo-ostium has to be created using a thermal cutter (Image 4). The author always begins with the left coronary artery re-attachment. This anastomosis is performed using a continuous 6.0 Prolene suture. A bovine pericardial strip is used only exceptionally in case of very friable coronary buttons or in case of aortic dissection. For the left-handed surgeon, it is rather easy to start this continuous suture from inside the coronary orifice and then from outside of the graft. The posterior part of the anastomosis is constructed and then the suture is changed. For the anterior part of this anastomosis, the suture is stitched from outside of the coronary orifice to inside-out of the vascular prosthesis (Image 5). When the left coronary orifice has been re-implanted, the same procedure is done for the right coronary artery and the anastomosis is performed in the similar way as the left side (Image 6): here the suture is stitched (for the back wall) from inside-out of the graft and thereafter from outside - in. Once both coronary anastomoses have been performed, the graft is pressurized using cold blood cardioplegia into the aortic root and cross-clamp for the graft. At this stage, some fibrin glue may be used to seal the small suture line.
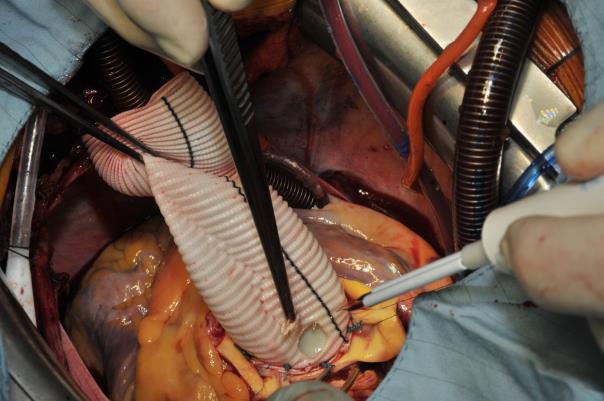
Image 4: Creation of the left neo-orifice to reattach the left coronary artery, using a thermal cutter.
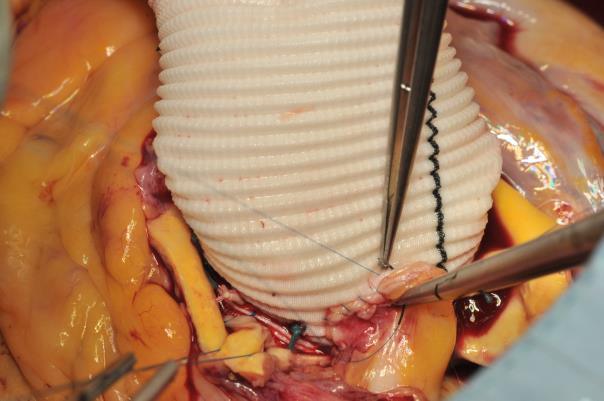
Image 5: The left coronary ostium has been re-attached. I start the anastomosis at the posterior wall from inside the coronary artery and stitch the graft from outside. Once half of the anastomosis has been performed, the author continues with the other end of the suture and perform the anterior wall from outside-in the coronary artery and inside-out through the graft (the last stitch is demonstrated in this image).
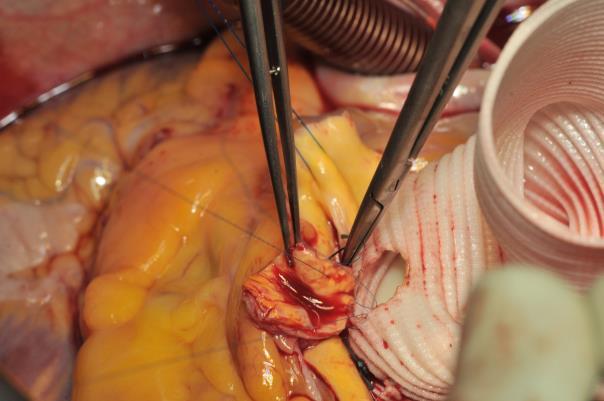
Image 6: Same procedure for the right coronary artery. The anastomosis for the left-handed surgeon is started on the back wall in-out through the graft and then the needle passed into the coronary button out-in. Once half of the anastomosis has been performed, the work is continued with the other end of the suture that is passed through the graft in-out and then through the coronary orifice.
With a minimal but not excessive mobilization of the coronary arteries, direct implantation of these buttons into the vascular graft is always possible. In some very few but complex instances of reoperation, an alternative technique of implantation of the coronary arteries may be necessary, if mobilization of the buttons may be hazardous. In these cases the author has used the classical Cabrol’s technique with a 6 to 8 millimeters prosthetic graft to re-implant the corresponding coronary artery. More recently, the author has preferred the use of a short segment of the saphenous vein for this purpose. Before ending the procedure, the proximal prosthesis of the composite graft (proximal part) is anastomosed to the prosthetic segment coming from the aortic arch using 4.0 Prolene continued suture line.
At this stage, the ascending aorta is de-aired using a needle and the cross-clamp is removed. The patient is still in Trendelenburg position and the de-airing is facilitated with the use of CO2 insufflation in the operating field (Image 7). Epicardial pacemaker electrodes are placed on the ventricle and the right atrium and the patient is weaned from cardio-pulmonary bypass under AAI-stimulation 90/min. as soon as the core temperature has reached 35.5 °C.
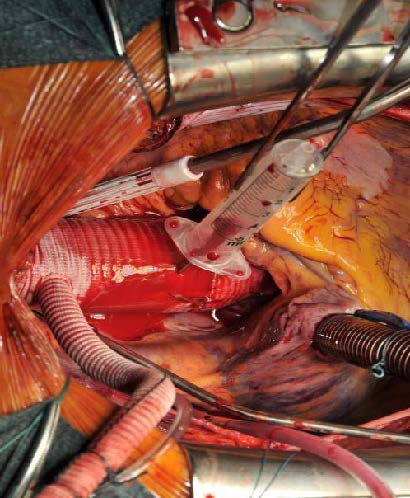
Image 7: ;Intraoperative situs at the end of the procedure. The composite graft prosthesis has been anastomosed with the proximal arch – ascending graft. A needle is used for de-airing. The patient is in Trendelenburg position. Left the anteflow side-arm with the arterial cannula for return from CPB.
The pericardium is closed over the prosthetic graft, and thoracic drains are put in the pericardial cavity and in the retrosternal space. The sternum is closed in typical fashion using wires. The skin is then closed intracutaneously with self-resorbing suture material. Before leaving the OP-theater, transesophageal echocardiography confirms the quality of the repair and checks the filling of the heart, the function of the valve, and the contractility of both ventricles.
Comments
Repair of the aortic root using the composite graft replacement is an established technique for patients in whom an aortic valve sparing root repair is not suitable. The composite graft is available with the best prosthetic material available on the market: bileaflet valves attached to vascular grafts that are used for isolated aortic replacement (1).
Since the original description by Bentall, numerous modifications of the technique have been suggested. The contemporary technique of coronary artery re-attachment using open or buttoned anastomoses was introduced by Nicholas Kouchoukos in 1981 (2). Material refinements have considerably improved the hemostasis at the level of the vascular graft and also at the junction of the valve sewing cuff with the vascular prosthesis. Therefore, the current method for composite graft is the open technique rather than the graft inclusion technique, which can be still recommended for the most complex cases (e.g. reoperations). In case of difficult hemostasis, a pericardial patch can be used to include the graft (the suture line passes from the pulmonary artery, the free upper margin of the right ventricle, and on the right side to the superior vena cava). If there is tension under the patch due to persistent bleeding, the space below the patch can be derivated into the right atrium using a small caliber graft as a modification of the technique described by Cabrol. With today’s improvements, this is extremely rare.
The experience with composite graft replacement in the author‘s institution represents approximately 80 cases per year with different pathologies (3). In the elective setting, hospital mortality has been comparable to the mortality of isolated aortic valve replacement and lies around 1.5 to 2.5%. The perioperative risk is increased in cases of aortic dissection but radical repair of the aortic root prevents later re-operations.
The fact that the operation is performed by a left-handed surgeon will not increase the perioperative risk (4). In his report on left-handed surgeons, Adsumilli revealed the perceptions of left-handed surgeons in adapting to a right-handed world (5). Personally, the author does not expect that left-handed surgeons need to adapt to techniques described by right-handed mentors. Early laterality related mentoring in medical school and during surgical residency with the provision of left-handed instruments may reduce the inconveniences that left-handed surgeons may encounter during learning. However, the author has found some situations to be facilitated by being left-handed, for instance the construction of the distal anastomosis on the open arch, as well as the re-attachment of the left coronary artery to the vascular part of the composite graft.
References
- Turina M. Composite Graft replacement of the aortic root using the button technique. Multi-Media Manual Cardio-thorac Surg 2003, doi: 10.1510/MMCTS.2003.000001.
- Kouchoukos NT, Karp RB. Resection of ascending aortic aneurysm and replacement of aortic valve. J Thorac Cardiovasc Surg. 1981;81:142-3.
- Paterick TE, Ammar KA, Jan MF, Loberg R, Buch M, Khandheria BK, Tajik AJ. Aortopathies: etiologies, genetics, differential diagnosis, prognosis and management. Am J Med 2013;126:670-8.
- Bann S, Darzi A. Selection of individuals for training in surgery, Am J Surg 2005;190:98.102.
- Adusumilli PS, Kell C, Chang JH, Tuorto S, Leitman IM. Left-handed Surgeons: Are They Left Out?. Current Surgery 2004;61:587-91




