ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
The Edge-To-Edge Technique For Barlow’s Disease
Patient Selection
The edge-to-edge technique is a method to treat mitral valve regurgitation by suturing the edges of the leaflets at the site of regurgitation. Although the method is technically simple, its application should follow a few rules to ensure effective and durable results, and to avoid complications.
The most critical issue is to avoid mitral stenosis, since the technique is always associated with sensible reduction of the valve area. This report will focus on technical hints and on clinical indications and contraindications to avoid this complication, in the subset of patients with Barlow's disease
The clinical results of the technique have been reported in the literature and are available through CTSNet. 1,2,3
The Concept
When there is an alteration of the motion of the edge of one leaflet, it can be corrected by suturing together the diseased edge to the normal one. This generates a double orifice valve if the lesion is located at the middle of one or both leaflets, and in a single orifice valve, with a smaller orifice area, when the suture of the leaflets is lateral. We called the first instance double orifice repair, and the second case paracommissural repair.
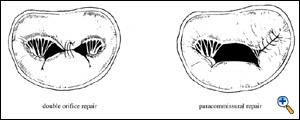 |
In Barlow's disease, the basic mechanism of regurgitation is the prolapse of both leaflets, with redundancy of valve leaflet tissue and subvalvular apparatus, associated with dilated annulus. Although both leaflets in systole reach the same level above the annulus, mitral regurgitation develops because coaptation surface is reduced by the excessive motion of the leaflet and by the associated annular dilatation. Moreover, the free edge of the myxomatous valve is often polylobular, with increased number of clefts in both the anterior leaflet and the posterior leaflet. Due to these malformations, and to increased tissue rigidity, mitral regurgitation is often complex, with more than one regurgitant jet, and with time variance of the degree and direction of the jets.
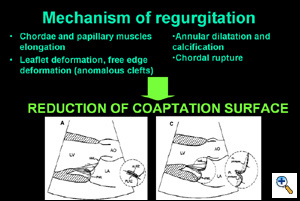 |
The effect of the edge-to-edge technique is to correct leaflet redundancy (which is usually predominant in the middle of the leaflets), to force coaptation (reducing the delay between the initiation of ventricular contraction and valve closure), to restrict leaflet motion and to prevent post-operative SAM.
Indications
The best application of the edge-to-edge is when the valve is severely affected by myxomatous changes, with leaflet thickening, bileaflet significant prolapse and redundancy, multiple jets at Colour-Doppler TEE.
 |
The presence of a flail leaflet is not a contraindication for the procedure, since the flail segment can be resuspended by the edge-to-edge method. In this case the location of the edge-to-edge suture will be determined by the position of the flail.
Small valve area is a contraindication for the edge-to-edge technique, since it is an increasing risk factor for postoperative mitral stenosis. However, in Barlow's disease, valve area is usually excessively wide, due to annular dilatation and to tissue redundancy.
Annular calcifications are not a contraindication for the edge-to-edge technique, however, when the annulus is widely and completely calcified, an annular ring prosthesis cannot be added to the procedure, and this has been associated with higher risk of late failure of the repair. We currently do not advocate the edge-to-edge repair as an isolated procedure in presence of severely calcified annulus.
Excessive leaflet fibrosis and rigidity is another contraindication (rare in the setting of Barlow's disease), since post-repair area depends on leaflet pliability.
Operative Steps
Conventional aortic and bicaval cannulation is performed. The interatrial groove is dissected to improve exposure, however, one of the characteristics of Barlow's disease is the pliability of the tissues, hence valve exposure is usually excellent. Cardioplegia is given and the left atrium is incised and entered laterally. The mitral valve is exposed with a Cosgrove mitral retractor. Inspection is carried on as usual. If the diagnosis of Barlow's disease is confirmed and prolapse is more than 1 cm beyond the annular level, the edge-to-edge repair is indicated. In most cases of Barlow's disease, 4-0 polypropylene suture (double armed prolene SH-1) is usually appropriate, since leaflets are thick and redundant. A 5-0 polypropylene suture may be preferable in case of thin tissues. Pledgets are not necessary.
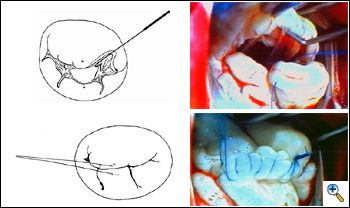 |
If there is a flail leaflet, the stitch may include the flail segment and corresponding leaflet opposite it. More often, however, there are no flail leaflets, but all portions of the valve prolapse. In this case, the stitch is positioned exactly at the “anatomical middle” of the valve. The anatomical middle of the valve can be defined as the point where the subvalvar structures converge in the middle of the anterior and posterior leaflets. This point divides the valve into two symmetric halves, each one connected to one papillary muscle. The anatomical middle of the valve may differ from the geometrical one, and usually is closer to the anterior than to posterior commissure because the distribution of the posterior papillary muscle chordae is wider than the anterior one.
The convergence of the chordae coming from the anterior and posterior papillary muscles is inspected with a nerve hook, and the suture is passed deeply into the anterior leaflet body at this site.
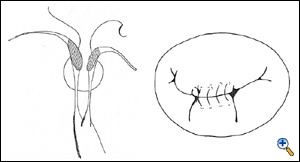 |
The same is done for the posterior leaflet (here the convergence is usually beneath the central scallop, P2, but sometimes accessory papillary muscle heads may be misleading, generating some risk of asymmetric stitching). Once the middle point has been identified, symmetry of the suture is checked, and suture is run towards the commissures as needed. Two rows of suture are placed, the first is continuous mattress and the second is over-and-over continuous suture.
 |
Stitch depth is approximately 0.5 to 1 cm from the free edge of the leaflets. However, stitch depth may vary, depending on the redundancy of leaflet tissue: as a general rule, the more redundancy is present, the deeper the stitch, and wider the suture. As a rule, the width of this suture should be minimized, as there is a trade-off between the extent of leaflet apposition and the risk of valvular stenosis. Suture width depends on two factors: degree of prolapse and valve orifice area. If valve prolapse and leaflet redundancy is more severe, a wider suture, connecting the whole P2 free edge to the opposing A2, may be necessary. In this case, however, valve area will be significantly decreased by the procedure, and this should be reserved only for those cases with a very large preoperative valve area. If there is any doubt regarding the post-repair valve area, the valve orifices should be probed with Hegar dilators to assess orifice areas. The total valve area should be greater than 2.5 cm.2
As with any other valve repair technique, annuloplasty is necessary to remodel and to reduce the size of the annulus which is usually very dilated in Barlow's disease. In our experience, when an annuloplasty was not performed, there was a higher incidence of late failure of the edge-to-edge repair.
Annuloplasty ring size is chosen as usual, by measuring the intertrigonal or intercommissural distance, and by measuring the width of the anterior leaflet surface area. We have used only rigid and semirigid rings in the setting of Barlow's disease. Annuloplasty may be avoided in case of calcified annulus, but long-term results in this setting are suboptimal.
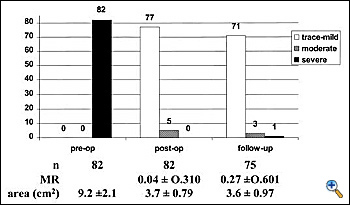 |
Intraoperative, post-repair TEE is mandatory, as with any other repair technique, to verify the results of the correction. In the specific setting of the edge-to-edge technique, TEE should exclude not only residual regurgitation, but also valve stenosis. Valve area may be assessed by Doppler methods, since flow velocity through the valve is not affected by the type of surgical procedure.4 We mostly rely on planimetric valve area, assessed in the transgastric, short-axis view of the mitral valve. In case of doubts, pressure measurements of the transvalvular gradients may be obtained to exclude mitral stenosis intraoperatively. Mid-term follow-up TEE data showed good results of the repair, with stable competence and no progression of valve stenosis (see chart3). Exercise Doppler-echocardiography may be useful in case of suspicion of mitral stenosis at baseline examination. It must be noted that following double orifice repair, valve area is flow dependent (functional reserve), since it increases during exercise, as for the native mitral valve.5
References
- Maisano F, Torracca L, Oppizzi M, et al. The edge-to-edge technique: a simplified method to correct mitral insufficiency. Eur J Cardiothorac Surg 1998;13:240-246.
- Alfieri O, Maisano F, De Bonis M, et al. The double-orifice technique in mitral valve repair: A simple solution for complex problems. J Thorac Cardiovasc Surg 2001;122:674-681.
- Maisano F, Schreuder JJ, Oppizzi M, Fiorani B, Fino C, Alfieri O. The double-orifice technique as a standardized approach to treat mitral regurgitation due to severe myxomatous disease: surgical technique. Eur J Cardiothorac Surg 2000;17:201-205.
- Maisano F, Redaelli A, Pennati G, Fumero R, Torracca L, Alfieri O. The hemodynamic effects of double-orifice valve repair for mitral regurgitation: a 3D computational model. Eur J Cardiothorac Surg 1999;15:419-425.
- Agricola, E., Oppizzi, M., De Bonis, M., Maisano, F., Toracca, L., Bove, T., and Alfieri, O. Multiplane transesophageal echocardiography performed according to the guidelines of the American Society of Echocardiography in patients with mitral valve prolapse, flail, and endocarditis: Diagnostic accuracy in the identification of mitral regurgitant defects by correlation with surgical findings. J Am Soc Echocardiogr 2003;16:61-66.




