ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Modified Fontan
Editors Note
Patient Selection
The completion Fontan operation is, as the name implies, the final stage of palliation for patients who have had previous surgery to address various types of single ventricle congenital heart disease. The first operation typically performed during infancy consists of techniques to regulate pulmonary blood flow (PBF) either with a pulmonary artery band when there is excessive PBF or a systemic-to-pulmonary artery shunt in the face of deficient PBF. Frequently, this first operation also includes techniques to relieve left-sided obstructive lesions and to ensure adequate mixing of systemic and pulmonary venous blood, as in the Norwood procedure. Alternatively, some patients with a single ventricle will be born with balanced pulmonary and systemic blood flow and may not require surgery during neonatal life. Following this initial surgery, an interval operation (or a primary operation for those who did not have surgery during infancy) is performed between 3 and 8 months of age. This procedure is designed to reduce the volume load on the functional single ventricle and thereby minimize the deleterious effects of ventricular hypertrophy combined with abruptly “unloading” the heart as may occur with an unstaged Fontan approach. This second stage consists of a superior cavopulmonary anastomosis constructed as a bi-directional Glenn, a hemi-Fontan, or occasionally a “classic” Glenn shunt. The completion Fontan is the third and final stage for single ventricle palliation and is performed typically between 18 months and 4 years of age depending on the method used.
Although the procedure known as the Fontan operation has undergone an evolution since its introduction in the late 1960’s, there are two modifications of the original operation that are the most widely used today. These modified Fontan operations are the lateral atrial tunnel Fontan and the extracardiac conduit Fontan. In both methods a total cavopulmonary connection is created, but by different means. With the lateral tunnel a baffle is placed in the right atrium to partition systemic from pulmonary venous blood. Because this partition comprises less than half the circumference of the pathway from inferior vena cava to pulmonary artery, the remaining native atrial tissue can grow to accommodate increased systemic venous return over time. The growth potential makes this type of Fontan operation suitable for smaller children without the need for future enlargement of the pathway. In the extracardiac conduit type of Fontan, one end of a synthetic tube graft is connected to the inferior vena cava and the other end to the pulmonary artery confluence. This pathway has obvious size limitations since the graft cannot grow. Therefore, extracardiac Fontan operation is performed in older children in order to implant a conduit size that will be suitable into adult life.
Prior to proceeding to a Fontan operation, a cardiac catheterization is performed to assess the suitability of the anatomy and hemodynamics for this type of circulation. Of particular importance are the adequacy of the pulmonary artery size and architecture, the presence of unobstructed pathways between systemic veins and pulmonary arteries, and low pulmonary vascular resistance (PVR < 2-3 Wood units). An echocardiogram is another important pre-Fontan test performed to assess systemic ventricular and AV valve function.
Operative Steps
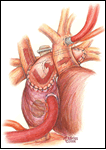
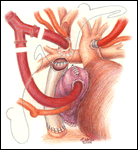
The child is positioned supine to perform the completion Fontan operation. An arterial line is inserted to monitor blood pressure and measure blood gases, but central venous access is not routinely used. Following endotracheal intubation, the ambient temperature is decreased, ice bags are placed on the head, and a cooling blanket is used, all to cool the patient. Through a median sternotomy approach dissection is performed to expose the aorta and right atrium initially. The patient is heparinized to achieve an ACT suitable for cardiopulmonary bypass followed by arterial cannulation of the ascending aorta and placement of a single atrial cannula for systemic venous drainage (Figure 1). Flow is established at 150 cc/kg/min, and then the perfusate is cooled to approximately 16o C. While the patient is cooled toward both nasopharyngeal and esophageal temperatures of 17o C, the remainder of the dissection is performed to expose adequate ascending aorta for cross clamping and to carefully free the right atrium from the adjacent pericardium. Once the desired patient temperature is reached, cardiopulmonary bypass flow is then temporarily stopped. The aorta is clamped and cold cardioplegia solution is infused to achieve an electromechanical cardiac arrest. Alternatively, this operation may be performed using moderate hypothermia and continuous circulatory support.
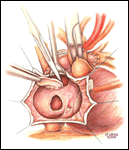
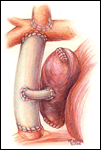
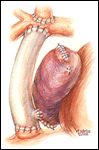
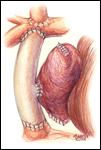
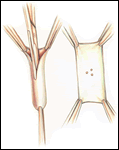
If a lateral tunnel cavopulmonary Fontan is preferred, the venous cannula is then removed and a right atriotomy is made in the atrial free wall parallel with and slightly anterior to the sulcus terminalis (Figure 2). The incision extends from the level of the eustachian valve inferiorly up to within a few millimeters of the patched orifice between the pulmonary artery and the right atrium that was constructed at the hemi-Fontan. This patch is then widely excised to create free passage from the right atrium into the pulmonary artery confluence opposite the previously constructed superior vena cava to pulmonary artery anastomosis. The lateral tunnel is created using a polytetrafluoroethylene (PTFE) baffle that is fashioned by longitudinally splitting a 10-mm tube graft (Figure 3) and sewing it within the atrium such that systemic venous blood returning through the inferior vena cava is directed into the pulmonary arteries. The suture line is started inferiorly where the PTFE is sewn to the perimeter of the inferior vena cava orifice using a 5-0 monofilament suture (Figure 4). Moving along the inferior and posterior right atrial endocardium, the suture line is carried cephalad staying inferior and rightward of the atrial septal defect. Superiorly, the baffle is sewn to the edges of the opening between the right atrium and the pulmonary arteries moving in a counterclockwise direction from posterior to anterior. The lateral tunnel is completed by sandwiching the remaining free edge of the baffle between the two sides of the atriotomy in the continuous suture closure (Figure 5). Most often we elect to fenestrate the baffle to allow the systemic venous pathway to decompress and enhance ventricular filling postoperatively. Creating three separate holes along the length of the PTFE graft using a 2.5- or 2.7-mm punch device typically configures these fenestrations. This step can be accomplished either when the graft material is cut prior to implantation or following the completion of posterior suture line between the baffle and atrial wall. The completion Fontan using the lateral tunnel technique as described here (Figure 6) can be accomplished with approximately a 20-minute period of hypothermic circulatory arrest.
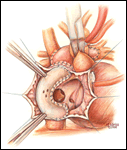
Upon completion of the atriotomy closure, the venous cannula is replaced into the atrium and cardiopulmonary bypass resumed. The flow is increased back to 150 cc/kg/min and the patient is actively rewarmed toward a nasopharyngeal temperature of 35o to 36o C. During rewarming ventilation of the lungs is done to optimize transpulmonary flow. Continuous ultrafiltration is used during periods of cardiopulmonary bypass with the aim to reduce net crystalloid administration. Modified ultrafiltration is used selectively. Low-dose inotropic support may be used to separate the patient from cardiopulmonary bypass. Once hemodynamic stability is achieved and the arterial blood gases are acceptable off circulatory support, the perfusion cannulas are removed. Fine caliber lines are inserted into the atrium via the cannulation site and secured with a purse string. These lines are used to monitor the cardiac filling pressure and as an access for infusions. We no longer monitor the cavopulmonary pressure as a routine. A single mediastinal chest tube is often sufficient for drainage.
Postoperatively the majority of patients can be extubated within the first eight hours. Inotropic support is typically continued through the first 24 to 72 hours. The mediastinal chest tube is removed when drainage is minimal; however, if significant pleural effusions develop, 8.5-Fr soft, pigtail catheters can be easily inserted. We routinely administer a baby aspirin, an angiotensin-converting enzyme inhibitor and diuretic daily. Most patients are discharged home within 10 days of surgery.
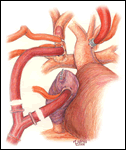
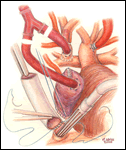
When preferred, the extracardiac conduit completion Fontan is a widely used alternative to the lateral tunnel procedure. Anatomic considerations such as pulmonary veins at risk for obstruction by a lateral tunnel configuration or systemic veins positioned posterior to an ipsilateral ventricular apex make the extracardiac conduit technique an appealing option. The preparation (Figure 7) and conduct of cardiopulmonary bypass and hypothermic circulatory arrest are the same as described for the lateral tunnel completion Fontan. Alternatively, continuous cardiopulmonary bypass with moderate hypothermia may be used. First, the inferior vena cava is transected 1 cm above the diaphragm and the cardiac end is over sewn (Figure 8). This step can be performed on circulatory support with the aid of cardiotomy suction in place of vena caval drainage, or after circulatory arrest. With the circulation arrested, a PTFE tube graft is connected to the inferior vena cava in an end-to-end anastomosis using a 5-0 monofilament suture. The conduit is then cut to the appropriate length with a slight bevel at the superior end (Figure 9). An anastomosis is constructed between this end and the pulmonary arteries. If the patient had a previous bi-directional Glenn, the conduit is spatially best suited to connect to the underside of the right pulmonary artery. However, in those patients who have had a prior hemi-Fontan operation, the conduit alignment is optimal if the superior anastomosis is created on the anterior surface of the pulmonary artery confluence through an opening in the homograft roof that covers the cavopulmonary connection from the previous surgery. Numerous methods of fenestration have been devised for an extracardiac conduit completion Fontan. A side-to-side anastomosis between the conduit and the atrium can be done directly or by placing a small intervening segment of PTFE tube graft (Figures 10 and 11). Alternatively, for patients who have had a prior hemi-Fontan a fenestration may be cut through the patch that occludes the pulmonary artery-to-right atrial connection (Figure 12).
Tips & Pitfalls
- Ensure that the opening from the right atrium into the pulmonary artery and superior vena cava confluence is as large as the inferior vena cava orifice.
- Avoid direct suturing to the sulcus terminalis because it has been implicated in the development of postoperative tachyarrhythmias.
- Create three small fenestrations (2.5-2.7 mm) in the PTFE baffle as opposed to one larger (4.0 – 5.0 mm) fenestration. Both will promote decompression of the venous pathway and enhance ventricular filling, but the smaller fenestrations are more likely to close spontaneously and not require device closure in the future.
- For construction of an extracardiac conduit a 16-mm PTFE tube graft is generally the smallest size suitable to accommodate inferior vena caval return into adult life.
- The preference for using a brief period of hypothermic circulatory arrest rather than accomplishing completion of the total cavopulmonary connection using continuous cardiopulmonary bypass is based in part on the goal of avoiding phrenic nerve injury. This nerve injury is potentially associated with repeated circumferential dissection and placement of tourniquets around the venae cavae.
Results
Table 1. Extracardiac and Lateral Tunnel Total Cavopulmonary Connection Fontan, 1992-2002
|
Author |
# Patients |
Type of |
Mean |
Mortality (%) |
|
Hospital |
||||
|
Jacobs1 |
72 (1996-2000) |
65 LT 7 ECC |
3.3 |
0 |
|
Gaynor2 |
332 (1992-9) |
281 LT 51 ECC |
_ |
6.6 |
|
Mosca3 |
100 (1992-5) (1995-8) |
100 LT |
_ |
11 (All HLHS) |
|
Azakie4 |
107 (1994-8) |
47 LT 60 ECC |
2.6 |
5.6 |
|
Kumar5 |
70 (1995-2002) |
37 LT 33 ECC |
3.3 |
2.8 |
|
Tokunaga6 |
100 (1994-2000) |
100 ECC |
3.1 |
0 |
Legend: CPB MH cardiopulmonary bypass with moderate hypothermia, DHCA deep hypothermic circulatory arrest, ECC extracardiac conduit, HLHS, hypoplastic left heart syndrome, LT lateral tunnel
References
- Jacobs ML, Pourmoghadam KK, Geary EM, et al. Fontan’s operation: Is aspirin enough? Is Coumadin too much? Ann Thorac Surg 2002;73:64-8.
- Gaynor JW, Bridges ND, Cohen MI, et al. Predictors of outcome after the Fontan operation: Is hypoplastic heart syndrome still a risk factor? J Thorac Cardiovasc Surg 2002;123:237-45.
- Mosca RS, Kulik TJ, Goldberg CS, et al. Early results of the Fontan procedure in one hundred consecutive patients with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2000;119:1110-8.
- Azakie A, McCrindle BW, van Arsdell G, et al. Extracardiac conduit versus lateral tunnel cavopulmonary connections at a single institution: Impact on outcomes. J Thorac Cardiovasc Surg 2001;122:1219-27.
- Kumar SP, Rubinstein CS, Simsic JM, Taylor AB, Saul JP, Bradley SM. Lateral tunnel versus extracardiac conduit Fontan procedure: A concurrent comparison. Ann Thorac Surg 2003;76:1389-9776.
- Tokunaga S, Kado H, Imoto Y, et al. Total cavopulmonary connection with an extracardiac conduit: Experience with 100 patients. Ann Thorac Surg 2002;73:76-80.
- Amin Z, McElhinney DB, Straun JK, et al. Hemidiaphragm paralysis increases postoperative morbidity after a modified Fontan operation. J Thorac Cardiovasc Surg 2001;122:856-62.