ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Post Coronary Bypass Surgery Angiography and Interventions
Movahed MR. Post Coronary Bypass Surgery Angiography and Interventions. March 2009. doi:10.25373/ctsnet.21355401
Patient Selection
Coronary angiography in the setting of coronary artery bypass grafting (CABG) is important diagnostic tool for the evaluation of bypass graft patency in patients presenting with angina or ischemia. Furthermore, coronary interventions in diseased vein grafts are common practice for the treatment of graft atherosclerosis. Angiography in the setting of CABG is more complex and challenging. Before the vein graft angiography is performed, it is very helpful to have the operative report available. It will assist the angiographer to know the exact number and anatomical features of the vein grafts. At least the types and number of vein grafts should be available before pursuing with angiogram. It can substantially decrease radiation exposure, procedural time, contrast use and the risk to the patient. For example, the knowledge about lack of internal mammary artery (IMA) utilization as a bypass graft conduit will eliminate the need for left or right subclavian artery catheterization with reduction in the risk, radiation exposure and contrast use. Available information about the number of grafts will eliminate the search for unknown number of grafts. It is important to know if the right coronary artery was grafted. Vein grafts to the right coronary arteries have usually right-sided take off from the aorta while left coronary grafts usually have an anterior take off. Sequential vein grafts supplying two coronaries with one vein graft are not uncommon. Therefore, the knowledge about the presence of sequential vein grafts will aid the angiographer to avoid searching for additional non existing vein graft ostium
The indication for performing coronary and vein graft angiography in patients with CABG is similar to the patients without bypass surgery. Unstable or symptomatic patients, who are candidates for coronary intervention, should undergo this procedure. Asymptomatic patients with a large area of ischemia, particularly with the new onset of left ventricular dysfunction or congestive heart failure, may benefit from angiography and intervention. It is important to discuss higher use of contrast and longer procedural time with the patient. Other limitations of coronary angiography are similar to the patients undergoing native coronary angiography. Patients with risk of bleeding, infection, peripheral vascular disease, renal failure, anemia, coagulopathy, congestive heart failure and significant co-morbid conditions are at higher risk for complications. It is important that the patient agrees to undergo revascularization if deemed necessary before pursuing with angiography.
Operative Steps
The sequence of angiography:
There is no clear guide of using specific sequence in performing vein graft and native vessel angiography. Performing angiography of the vein graft first, has the advantage of obtaining important information about the number of occluded vein grafts guiding the operator to focus on the native coronaries that were supplied from the occluded grafts. Furthermore, the knowledge about patency of all grafts will decrease the need for performing extensive angiography in the native coronaries potentially saving contrast and radiation. On the other hand, performing native coronary angiography first, have the advantage of visualizing distal vein graft insertion site or competitive flow that can be seen from native coronaries suggesting patent grafts. This is more helpful when the operator does not have accurate information about the numbers and types of vein grafts before performing angiography.
Arterial access:
Similar to native coronary angiography, femoral, radial and brachial arteries can be used for grafts angiography. However, in the presence of left internal mammary artery (IMA), if upper extremities are used, left arm should be utilized in order to have easy access to the left IMA and vein grafts. For the presence of right IMA, right arm should be selected if necessary. Arterial access is obtained using a modified Seldinger technique by anterior puncture of the artery. Due to smaller arterial size for radial or brachial artery, a micro puncture system could initially be used.
Brachial access site is associated with a higher risk of thrombotic complications. Therefore, intra-arterial injection of 5000 units of heparin is routinely given after the sheath insertion. Radial artery is proned to spasm. Intra-arterial injections of nitroglycerin, verapamil and heparin are usually used after the sheath insertion into the radial artery. Radial artery is small which cannot accumulate large sheath size. Usually a 5 French sheath is used for diagnostic and a 6 French sheath for interventional proposes. Before radial access site is considered, an Allen test has to be performed to ensure ulnar artery patency. Radial artery occlusion is usually asymptomatic in patients with patent ulnar artery without any need for intervention.
Manifold:
Once the sheath is inserted and flushed with saline solution, a diagnostic or interventional guide catheter is advanced over the 0.035 inch J wire into the ascending aorta. After the removal of the wire, the catheter is connected to the manifold and double-flushed (aspirating blood followed by flushing with heparin saline) vigorously with the removal of all bubbles. Next, contrast is drawn into the injecting syringe and pressure is monitored during the entire procedure.
Anatomical localization of the vein grafts ostia:
In the past, surgeons used rings to mark the ostia of the vein grafts substantially aiding the angiographer to localize the vein graft ostia during angiography. However, nowadays, it is not a usual practice. The lack of marker can increase the contrast use and radiation particularly in patients without the knowledge of the types and number of vein grafts. As mentioned earlier, right coronary grafts have usually a right sided take off from the aorta. Therefore, using standard view, left anterior oblique (LAO), which is used for right coronary artery catheterization is the view of choice. Left coronary bypass grafts have usually anterior take off. Therefore, a right anterior oblique (RAO) makes it easier to engage the left-sided vein graft ostia preventing foreshortening of the catheter tip. Using RAO, the catheter tip should be oriented to the right side of the screen. The vein grafts to the left system is based on the anatomical proximity of the native coronary to the aorta. Therefore, LAD graft ostia are usually closest grafts to the aortic valve followed by diagonal and circumflex grafts. Circumflex graft ostia usually have the highest take off from the aorta. There are occasional cases with different take off making vein graft angiography difficult. In such a situation, the angiography catheter has to be probed across the aorta in different level in order to engage the ostial vein graft. A non-selective strong contrast injection or aortogram may be necessary to delineate the unusual take off of missing vein grafts or documenting total occlusion of missing vein grafts. Total occluded vein grafts usually have a residual knob in the aorta that can be seen during angiography.
Technical aspect and catheter choice:
Initially, after gaining access, catheters are exchanged over the wire. Once the catheter crosses the aortic arch, the J wire is removed and the catheter is flushed thoroughly to remove any micro thrombus or air bubbles. Next, appropriate views are selected. Graft catheterization is performed by advancing and rotating the catheter until it engages the graft ostium. It is important to watch for any damping or ventricularization of the aortic pressure. These would signify a high grade ostial lesion or catheter touching the vessel wall. It is important to avoid injecting into the vessel wall as it can cause major dissection. Injection into the vein graft with a high grade ostial lesion increases the risk of arrhythmias.
Catheters:
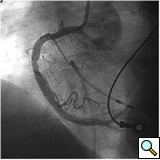
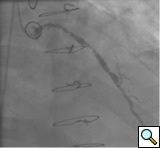
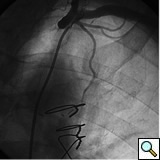
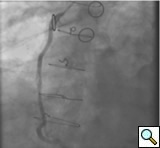
The most commonly used catheters for left heart catheterization and vein graft angiography can be seen in Figure 1 and Figure 4-13. Most of the vein grafts have horizontal take off and can be successfully engaged using a commonly used Judkins right number 4 (JR4) catheter. The JR4 catheter is the most commonly used catheter for the engagement of the right coronary ostium with horizontal take off. However, many vein grafts have unusual take off requiring different catheters. Many right coronary vein grafts have steep inferior take off making the ostial engagement with JR4 difficult or impossible (Figure 10). In such a scenario, a multipurpose catheter which has a shallow angulation is the best choice (Figure 11). The second major challenge in engaging vein graft ostia, particularly vein grafts supplying the left coronary arteries, is the shape of the aorta. A large aorta can make it very difficult for the JR4 catheter to reach the ostial vein grafts. In such a situation, Amplatz (AR) right and left (AL) catheters can be very helpful to reach the vein graft ostia. Amplatz catheters have a larger primary curve and have been used successfully in unusual superior take off of left coronary arteries or vein grafts and in large aorta. Amplatz catheters are available in different sizes (from smaller to larger curve: AR 1, AR2, AL2, AL2 and AL3). Occasionally, a very superior take off of a vein graft requires specially designed bypass graft catheters. Amplatz catheters are also extremely helpful in engaging native right coronary ostium with anterior take off.
Arterial graft angiography:
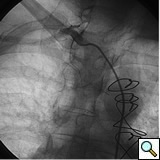
For the left IMA angiography, there are two major challenges that need to be overcome. First, wire and catheter advancement into the subclavian artery can be difficult. Older age and peripheral vascular disease are the risk factors for tortuous anatomy. Subclavian stenosis is another cause of difficult subclavian catheterization. In most instances, a JR4 catheter can be positioned into the subclavian artery ostium by counter-clockwise rotation and withdraw of the catheter after positioning it in the ascending aorta. Once the catheter is engaged in the subclavian artery ostium, any manipulation of the catheter has to be performed with extreme caution in order to avoid injury to the subclavian artery and embolism in the territory of vertebral artery. If the catheter advances without any difficulty, it will be reasonable to advance the JR4 catheter and try to engage the left IMA. However, due to steep take off of the left IMA, usually only a non-engaged angiography can be performed using this catheter. It is reasonable to inflated a blood pressure cuff in the left arm and perform a non-selective angiography of the left IMA. In the majority of cases, IMA opacification is satisfactory (Figures 6 and 7). However, for unsatisfactory opacification and when further detail of anatomical information of IMA is needed, a JR4 catheter needs to be exchanged to a left IMA catheter using a long exchange J tip wire. Exchange wire can also be used earlier after subclavian engagement particularly when a JR4 catheter can not be advanced easily. In the majority of cases using left IMA catheter, excellent engagement and angiography of the left IMA can be performed. Again, it is important to avoid extreme manipulations of any catheters in the subclavian artery to avoid any vascular injury and embolism. Contrast injection in the IMA can trigger severe pain in the arm. The patient needs to be warned and informed before injecting the contrast. If subclavian ostial engagement cannot be achieved with a JR4 catheter, a J-wire could be utilized and positioned into the subclavian artery followed by the left IMA or JR4 catheter advancement. There are rare instances when subclavian engagement can not be achieved from femoral arterial route. In such a situation, using the left radial or brachial artery gives direct access into the left IMA ostia. The technique for engaging right IMA is similar to the left IMA. However, right IMA angiography and engagement can be more difficult in dilated aortic root and abnormal steep take off of the right innominate artery. Similar to the left IMA, the right arm can be used in difficult cases. Radial artery graft angiography has the additional challenge of inducing spasm which may require intra-arterial injection of nitroglycerin for appropriate assessment of radial arterial graft size and patency. Aortogram and left ventriculography are usually performed using a pigtail catheter and power injector for the assessment of the left ventricular function, aortic valve regurgitation or missing vein grafts.
Special issues related to vein graft angiography:
- Higher contrast use with increasing risk of contrast induced nephropathy
- Increased radiation exposure to the patients and angiographers
- Longer procedural time
- Higher risk for thromboembolism and aortic injury during additional catheter manipulations in the aorta
- Risk of injury to the subclavian artery and aorta during IMA angiography
- Difficulty in engaging angulated vein grafts take off and subclavian artery in some patients.
Percutaneous Coronary Intervention (PCI) of the Vein Grafts or IMA
Importance of guide support:
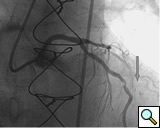
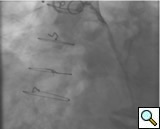
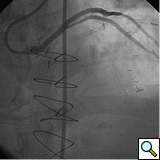
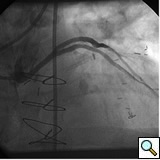
PCI of the vein and arterial grafts have unique challenges. For any PCI, guide support is very important for successful balloon and stent delivery. In a tortuous vein graft with a steep angle, advancement of a stent can be very difficult and challenging. Therefore, it is important to choose the best available catheter before starting PCI. Similar to the right coronary angiography, a JR4 guide catheter is most commonly used in this setting. However, Amplatz guide catheters for left vein grafts and multipurpose catheters for right vein grafts are better choices in certain anatomy. In Figure 8 and Figure 10 two examples of poor guide support in two vein graft interventions can be seen. Initially, a JR4 guide was used for PCI of the vein graft supplying the left anterior descending artery (LAD) without any success. However, after changing the guide to an Amplatz left 2 guide catheter, we achieved excellent support without any difficulty in advancing two stents (Figure 9). In Figure 10, difficulty is illustrated in engaging the vein graft ostium supplying the right coronary artery with a JR4 catheter. This vein graft has a very steep inferior take off from the aorta. After changing the guide to a multipurpose catheter, we were able to deliver three stents successfully without any difficulties (Figure 11). Similar challenges exist in the treatment of the left IMA or right IMA. These arterial grafts can be extremely tortuous making stent delivery very difficult. It may be necessary to use short length stents for a better deliverability or stents with lowest profile. Usually, similar to the native coronary intervention, a 6 French guide is appropriate for the routine use.
Wires:
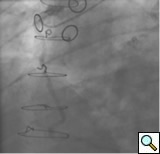
Similar to the native coronary, a 0.014 inch wire is usually used for balloon angioplasty or stenting. If the stent can not be advanced using a soft wire, the wire could be exchanged over the balloon to a stiffer wire or the second wire could be advanced and used as a body wire for a better support. If possible, filter wires or other protection devices should be used in all vein graft interventions [(Figure 2, see no-reflow chapter for details. Most commonly used work horse wires are balanced mid weight (BMW Boston Scientific), all track wire (ATW Cordis) or Prowater (Abbott Vascular)]. In the tortuous vessels, it may be necessary to exchange the wire over a balloon to a stiffer wire such as platinum plus or an iron man. Additional wire for support so called” body wire” can be utilized to improve stent delivery in tortuous vessels. Similar to the native coronary angioplasty, wire with stiffer or/and hydrophilic tips can be used for crossing a high-grade lesion such as PT choice, grand slam, PT2 wires, etc. However, these wires can easily enter a false lumen causing dissection or perforation. It is advised to exchange these wires with a softer tip wire once the lesion is crossed successfully to prevent distal vessel perforation.
Balloon angioplasty and stenting:
In order to minimize distal embolization, primary stenting without previous angioplasty is recommended in vein graft interventions. However, in angulated and very high grade lesions, stent without balloon angioplasty may not be able to cross a lesion requiring balloon angioplasty before stenting. Balloons should be sized smaller in order to prevent rupture or dissection. Once an angioplasty balloon is advanced across the lesion, usually it is inflated to 6 or 8 atmospheres for few seconds. Stenting is similar to the balloon. Once a stent is placed across the lesion, it is inflated to higher pressures such as 12-14 atmospheres. The use of a bare metal or drug eluting stents in vein grafts is controversy. This topic is discussed in detail in the restenosis chapter. Angioplasty and stenting of bifurcation sequential vein grafts at the site of the bifurcation or distal to the graft is challenging and usually requires more complex interventions including double wiring and utilization of two stents. A comprehensive classification with guide to interventional techniques for bifurcation lesions is published recently that can be used as a guide for bifurcation lesions interventions involving vein grafts [1].
No reflow:
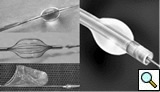
One of the most common complication of PCI during vein graft interventions is the occurrence of “no-reflow.” After angioplasty or stenting of a vein graft, sudden cession of the distal flow could occur. This phenomenon thought to be secondary to distal embolization and platelet aggregation [2-4]. It can cause myocardial infarction, arrhythmias, hemodynamic collapse and death. It is usually treated with intra-coronary infusion of adenosine, calcium channel blockers, or nitroprusside [5-9]. After aggressive pharmacological treatment, it is usually reversible but it can be refractory causing severe myocardial damage and infarction. It usually occurs in old degenerative vein grafts or in thrombus containing lesions. However, based on the angiographic appearance, it is hard to predict which vessel is proned to this complication. There are few strategies developed in order to prevent this complication. Intra-venous glycoprotein IIb/IIIa inhibitors, which are very potent platelet aggregation inhibitors, despite success in the native coronary interventions have failed to decrease this complication in vein grafts PCI [10, 11]. On the other hand, distal filter devices have shown to significantly reduce the occurrence of this complication in vein grafts and should be routinely used if technically feasible [12-14]. A major limitation of distal filter devices is the absence of large distal vessels that can accommodate these devices and difficulty to advance such a bulky device across a high-grade lesion or across a tortuous vein graft. A recently approved proximal occlusion device has similar efficacy for the prevention of no-reflow in vein graft interventions [15]. By occluding the vein graft proximally during balloon angioplasty or stenting, flow can be stopped during stent expansion and atherosclerotic and thrombotic debris can be suctioned before the vein graft flow is restored preventing distal embolization. This technique requires lesions that are distal to the ostial vein graft and add substantial complexity and time to the procedure. Figure 2 shows the most commonly used protection devices for vein graft interventions.
Recently, pretreatment of a vein graft with intragraft injections of 200 microgram nicardipine before PCI has substantially reduced the occurrence of this complication [16]. Risk of rupture is also increased in old degenerative vein grafts. Avoidance of stent or balloon oversizing is essential to decrease this potentially deadly complication. Arterial grafts have distinct advantages that no-reflow is less common in comparison to the vein graft interventions.
Stent restenosis and choice of stents:
For unknown reasons, vein graft angioplasty and stenting is associated with much higher risk of stent restenosis that can be as high as double in comparison to native coronaries. The use of drug eluting stents in the native coronary has dramatically decreased the risk of stent restenosis to less than 10% and is now a common practice. However, the use of drug-eluting stents in the setting of vein graft interventions has not been studied in a randomized trial. Current available limited data is very conflicting in regards to adverse outcome. Although the restenosis rate appears to be lower using drug-eluting stents with decrease in the major adverse outcome [17-18], a recent non randomized study suggested higher risk for mortality using drug-eluting stents in the vein grafts [19]. Others found no differences in the outcome [20]. Furthermore, large vein graft limits the use of DES as larger DES of over 4.0 mm in diameter are not currently available in the USA.
Early stenosis of vein graft or IMA anastomosis is a unique entity related to surgical suture and edema. In this setting, usually plain angioplasty is sufficient to produce satisfactory long term results. Furthermore, stenting in the setting of recent surgery increases the risk of perforation. The use of anticoagulation during vein graft intervention is similar to the native coronary PCI including heparin or bivalirudin, aspirin, clopidegrol and optional glycoprotein IIb/IIIa inhibitor use. There are other devices that have been tested for vein graft interventions such as rheolytic thrombectomy, laser or the use of self-expanding stents with limited success and are rarely used in the clinical practice.
Special issues related to PCI of vein and arterial grafts:
- Higher risk for distal embolization and occurrence of no-reflow during balloon angioplasty and stenting
- Higher risk of vein graft rupture in old degenerative vein grafts
- Higher risk of restenosis after balloon angioplasty and stenting
- Conflicting data in regards to the use of drug-eluting stents in the vein grafts
- Difficulty in having good guide catheter support in tortuous vein grafts or IMA
Preference Card
Sheaths:
5-8 F sheaths (Cordis, Inc., Miami, FL) (Terumo 5-8 fr x 10cm Pinnacle Sheath) is used for initial femoral artery access.
Micro-Puncture Introducer Set (Cook, Inc. 4fr, MPIS-401).
Catheters:
5-6F pigtail catheter (Cook, Inc. 5-6fr x 90cm Pigtail-Royal Flush Plus) used for ventriculography or aortogram.
3.5 F IVUS catheter (Volcano, 3.5x50 Eagle eye Gold).
5-7 F Judkins right 4, or 5 for right coronary or graft angiography or intervention, Guide or diagnostic catheters, Viking for guide, Abbott Vascular intervention .
5-7 F Judkins left 3.5, 4, 4.5 or 5 for left main engagement or left sided native coronary intervention, Guide or diagnostic catheters, Viking for guide, Abbott Vascular.
5-7 F Amplatz Right 1 or 2 for native or vein grafts. Guide or diagnostic catheters, Viking for guide, Abbott Vascular.
5-7 F Amplatz Left 1, 2 or 3 for native or vein grafts. Guide or diagnostic catheters, Viking for guide, Abbott Vascular.
5-7 F Multipurpose catheter for native and right sided vein grafts. Viking for guide, Abbott Vascular.
5-7 F LIMA catheter for left IMA. Guide or diagnostic catheters, Viking for guide, Abbott Vascular.
5-7 F Left Coronary bypass catheter Guide or diagnostic catheters, Viking for guide, Abbott Vascular.
5-7 F Right coronary bypass catheter Guide or diagnostic catheters, Viking for guide, Abbott Vascular.
Guide wires:
0.035 Fixed core wire guide safe-T-J curved (Cook Inc.).
0.035 (or 0.025) x 260cm Stiff type guide wire with soft tip (Boston Scientific 0.025 x 260cm.
Hydrophilic guide wire (Terumo 0.035 x 180cm Glidewire).
Interventional wires:
Soft tips:
0.014 x190 or 300 Balanced Middle Weight wire (Boston Scientific).
0.014 x180 or 300 Prowater Flex (Abbott Vascular).
0.014 x190 or 300 High Torque Floppy (Boston Scientific).
0.014 x 190 or 300 All Track Wire (Cordis Inc.).
Support wires:
0.014 x 190 or 300 Platinum Plus (Boston Scientific).
0.014 x 180 or 300 ASAHI Grand Slam (Abbott Vascular).
0.014 x 300 Ironman (Boston Scientific)
Protection Devices:
Percu-Surge (Guardwire, Medtronic).
Angioguard XP (Cordis).
Filterwire EX (Boston Scientific).
The Proxis Embolic Protection System (St. Jude Medical).
Balloons:
Maverick 1.5-4.0 x 9 or 15 or 20 mm angioplasty balloon (Boston Scientific).
Voyager 1.5-4.0 x 8, 15, 20 and 30 mm (Abbott Vascular).
Stents:
Bare metal stents:
3.0- 4.5 x 8, 12, 15, 18, 23, 28 mm Vision (Abbott Vascular).
2.0-2.5 x 8, 12, 15, 18, 23, 28 mm Mini-Vision stents (Abbott Vascular).
3.0-4.0 x 9, 12, 15,18,24, 30 Driver MX2 (Medtronic).
Drug eluting stents:
2.5-3.5 Taxus Express (Boston Scientific).
2.5-3.5 Cypher (Cordis).
2.5-4.0 Endeavor (Medtronic).
Miscellaneous:
Luer-Lock (Boston Scientific FloSwitch HP).
Needle for initial arterial access (Boston Scientific Arterial Entry Needle 18 gauge, 2 ¾ in).
Torque device ( Terumo Torque Device).
References
- Movahed MR, Stinis CT. A new proposed simplified classification of coronary artery bifurcation lesions and bifurcation interventional techniques. J Invasive Cardiol 2006;18:199-204.
- Engler RL, Schmid-Schonbein GW, Pavelec RS. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol 1983;111:98-111.
- Eeckhout E, Kern MJ. The coronary no-reflow phenomenon: a review of mechanisms and therapies. Eur Heart J 2001;22:729-39.
- Movahed MR, Butman SM. The pathogenesis and treatment of no-reflow occurring during percutaneous coronary intervention. Cardiovasc Revasc Med 2008;9:56-61.
- Fischell TA, Carter AJ, Foster MT, et al. Reversal of "no reflow" during vein graft stenting using high velocity boluses of intracoronary adenosine. Cathet Cardiovasc Diagn 1998;45:360-5.
- Fugit MD, Rubal BJ, Donovan DJ. Effects of intracoronary nicardipine, diltiazem and verapamil on coronary blood flow. J Invasive Cardiol 2000;12:80-5.
- Kaplan BM, Benzuly KH, Kinn JW, et al. Treatment of no-reflow in degenerated saphenous vein graft interventions: comparison of intracoronary verapamil and nitroglycerin. Cathet Cardiovasc Diagn 1996;39:113-8.
- Michaels AD, Appleby M, Otten MH, et al. Pretreatment with intragraft verapamil prior to percutaneous coronary intervention of saphenous vein graft lesions: results of the randomized, controlled vasodilator prevention on No-Reflow (VAPOR) Trial. J Invasive Cardiol 2002;14:299-302.
- Piana RN, Paik GY, Moscucci M, et al. Incidence and treatment of 'no-reflow' after percutaneous coronary intervention. Circulation 1994;89:2514-18.
- de Lemos JA, Antman EM, Gibson CM, et al. Abciximab improves both epicardial flow and myocardial reperfusion in ST-elevation myocardial infarction. Observations from the TIMI 14 Trial. Circulation 2000;101:239-43.
- Roffi M, Mukherjee D, Chew DP, et al. Lack of benefit from intravenous platelet glycoprotein IIb/IIIa receptor inhibition as adjunctive treatment for percutaneous interventions of aortocoronary bypass grafts: a pooled analysis of five randomized clinical trials. Circulation 2002;106:3063-67.
- Stone GW, Rogers C, Hermiller J, et al. Randomized comparison of distal protection with a filter-based catheter and a balloon occlusion and aspiration system during percutaneous intervention of diseased saphenous vein aorto-coronary bypass grafts. Circulation 2003;108:548-53.
- Gick M, Jander N, Bestehorn HP, et al. Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation 2005;112:1462-69.
- Huang Z, Katoh O, Nakamura S, Negoro S, Kobayashi T, Tanigawa J. Evaluation of the PercuSurge Guardwire Plus Temporary Occlusion and Aspiration System during primary angioplasty in acute myocardial infarction. Catheter Cardiovasc Interv 2003;60:443-51.
- Mauri L, Cox D, Hermiller J, et al. The PROXIMAL Trial: proximal protection during saphenous vein graft intervention using the Proxis Embolic Protection System: a randomized, prospective, multicenter clinical trial. J Am Coll Cardiol 2007;50:1442-49.
- Fischell TA, Subraya RG, Ashraf K, Perry B, Haller S. "Pharmacologic" distal protection using prophylactic, intragraft nicardipine to prevent no-reflow and non-Q-wave myocardial infarction during elective saphenous vein graft intervention. J Invasive Cardiol 2007;19:58-62.
- Lee MS, Shah AP, Aragon J, et al. Drug-eluting stenting is superior to bare metal stenting in saphenous vein grafts. Catheter Cardiovasc Interv 2005;66:507-11.
- Vermeersch P, Agostoni P, Verheye S, et al. Randomized double-blind comparison of sirolimus-eluting stent versus bare-metal stent implantation in diseased saphenous vein grafts: six-month angiographic, intravascular ultrasound, and clinical follow-up of the RRISC Trial. J Am Coll Cardiol 2006;48:2423-31.
- Vermeersch P, Agostoni P, Verheye S, et al. Increased late mortality after sirolimus-eluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC Trial. J Am Coll Cardiol 2007;50:261-67.
- Bansal D, Muppidi R, Singla S, et al. Percutaneous intervention on the saphenous vein bypass grafts - long-term outcomes. Catheter Cardiovasc Interv 2008;71:58-61.
Disclaimer
The information and views presented on CTSNet.org represent the views of the authors and contributors of the material and not of CTSNet. Please review our full disclaimer page here.