ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Scheie's Syndrome: Quite Rare, Quite Predictable
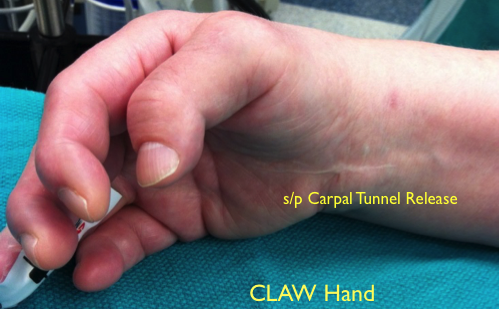
Fig. 1: Right Hand: short, spindle shaped fingers. Status post carpal tunnel release.
Introduction
An autosomal recessive lysosomal storage disease, Scheie’s syndrome is a hereditary disorder involving a missing lysosomal alpha-L-iduronidase enzyme used in the breakdown of glycosaminoglycan (GAG). Unbroken GAG can be deposited anywhere in the body, including: bones, cartilages, eyes, and the cardiovascular system (1). Unbroken GAG deposited in the heart can lead to diastolic, systolic, and valvular dysfunction (2). Early diagnosis, complete cardiac evaluation, and surveillance play an important role in altering disease progression and early mortality prevention.
Case Report
A 58-year-old mother of two complained of progressive congestive heart failure symptoms over the past three years, secondary to her multivalvular problems. At age 22, joint contractures and ophthalmologic complications led her to undergo a battery of tests confirming her diagnosis of MPS - 1 S. She had been on Laronidase for 1.5 years and that seemed to slow down her disease and symptoms. She had mild coarse craniofacial features and a short neck with slightly thickened skin, but had reasonable range of motion. She had a right eye implant and a legally blind left eye due to glaucoma. Her elbows, knees, and ankles were relatively normal on extension, despite her multiple joint contractures (Fig. 1). Oral and abdominal examinations were unremarkable.
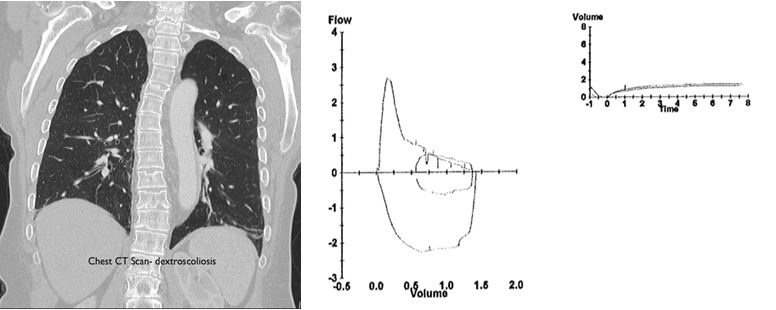
Fig. 2: Chest CT scan - dextroscoliotic. Spirometry: FEV 10.83L (37%), FVC 1.43L (50%), FEV1/FVC 58%, TLC 2.73L (62%), FRC 1.46L, DLCO 6.9 ml (31%), MIF 48 cmH20 (64%), MEF 52 cmH20 (37%)
The patient's resting room air Sp02 was 94%. Her pulmonary function test showed a mixed obstructive and restrictive pattern with severely impaired diffusion capacity (Fig. 2). She had a BP 116/82, HR 80 NRRR, RR 22 non-labored, height of 154 cm, weight of 74 kg, and BMI 31.1. Her lungs were clear. She had a 3-4/6 systolic murmur over the left parasternal border with occasional radiation to the axilla and no diastolic murmur. An echocardiogram (Fig. 3) showed a left ventricular EF of 65%, mild concentric LVH, moderate MR, severe AS, mild AI, and mild pulmonary hypertension. A right heart catheterization showed RAP 6, PAP 38/2, PCWP 18, LVEDP 10, AV gradient 30, and AVA 0.7 cm2. Her coronary arteries were free of significant angiographic stenotic or occlusive disease.
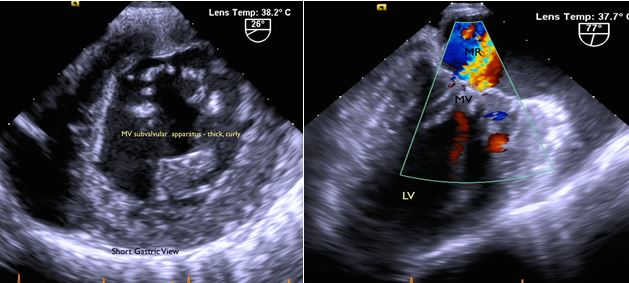
Fig. 3A: Mitral valve leaflet: thickened, redundant. Moderate central regurgitation. Subvalvular apparatus: nodular, thickened, fused.
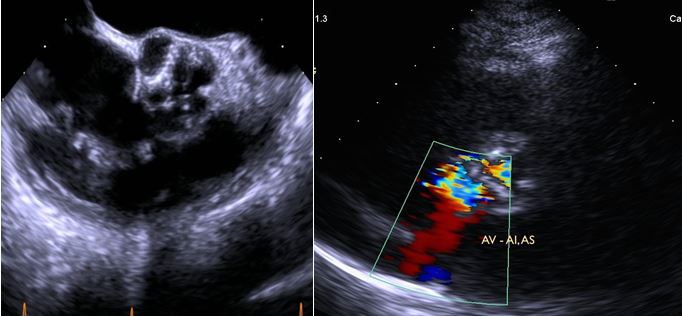
Fig. 3B: Aortic Valve: thickened, nodular, calcified valve. Severe AS and mild AI.
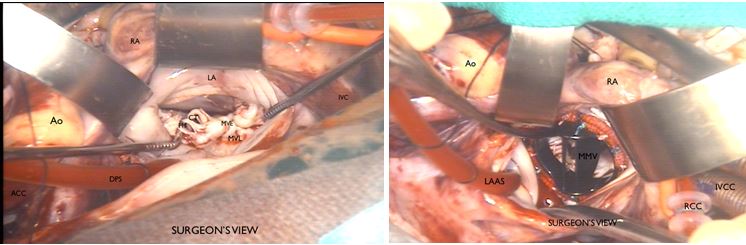
The patient agreed to proceed with the recommended double valve surgery. During the cardiopulmonary bypass, the antegrade cardioplegic solution was only allowed to run for one minute and was switched to retrograde for three minutes. The cardioplegic solution was blood crystalloid with 4:1 composition. Retrograde cardioplegia was given every 20 minutes for the rest of the operation. The heart arrested promptly and nicely with no distention noted. An aortotomy was carried out and spiraled into the noncoronary sinus exposing a heavily calcified aortic valve. The left and right coronary leaflets were conjoined. The aortic leaflet was excised and the deeply scalloped annulus was decalcified and debrided carefully with rongeurs. The aortic root measured 21 mm. The mitral valve was exposed via a left atriotomy through the right superior pulmonary vein junction and extending behind both cavae (Fig. 4).
Fig. 4: Mitral valve: thickened, myxomatous, short chordae tendinae. Mechanical mitral valve placed in leaflet.
The valve was beyond repair. Most of the anterior leaflet was excised and the commissural support was left intact. The mitral valve was replaced with a 27 mm SJM with no untoward problems. The previously sized aortic root of 21 mm was only able to accommodate a size 19 mm SJM valve for proper seating. Intraoperative testing showed well-seated and functioning valves, complimented by intraoperative TEE.
The patient was weaned from the cardiopulmonary bypass once parameters were met without any pharmacologic support or incidents. The total pump time was 179 minutes, with an aortic cross clamp time of 152 minutes. She was extubated within 24 hours with no untoward problems. Her postoperative period was unremarkable except for one episode of atrial fibrillation, which converted to sinus rhythm after one dose of beta blocker. She was discharged to home after seven days. She is now 3.5 years out from her surgery and is in good clinical condition.
Discussion
Mucopolysaccharidosis type 1-S (MPS 1-S), or the Scheie's variant, is the mildest form of all MPS (3). Affected patients have normal life span, intelligence, and height but with mild to moderate skeletal, pulmonary, and cardiac complications. Though this disorder is quite rare, the prevalence and severity of cardiovascular disease is 60-100% (4). The prominent valve component of normal valve tissue are the dermatan-sulfated GAGs and heparan-sulfated GAGs. These GAGs are dependent on a-L-iduronidase enzyme for breakdown, which unfortunately is missing in these patients.
In addition, valvular interstitial cells (otherwise known as clear, gargoyle, or Hurler cells residing within the cardiac valves) are rendered non-functional in repairing defective valve tissue. The left-sided valves, predominantly the mitral followed by the aortic, the tricuspid, and the pulmonary are affected in that order. The pathologic mitral valve is symptomatic in about 42%, with markedly thickened and cartilage-like leaflets and edges. The normal value for mitral valve leaflet thickness is 0.7-3.0 mm. The aortic valve leaflet thickness ranges from 0.7-3.0 mm, and some consider it pathologically thick if it is greater than 2.0 mm (4). The subvalvular apparatus has shortened chordae tendinae and the papillary muscles are thick and elongated (5). Calcifications are also prominent on the mitral annulus.
Fig. 5: Discharge CXR.
The aortic valve exhibits the same slow and progressive valvular dysfunction and becomes symptomatic in 20% of cases. Reports showed that 80% of patients with MPS I have thickened aortic valves with associated dysfunction. Regurgitation is more common than stenosis, though both pathophysiologies can coexist.
The ECG profile may exhibit a normal sinus rhythm, arrhythmia, or a QRS complex voltage that is either increased or decreased. The small QRS can either be due to GAG deposition in the interstitium or poor conductance of the GAGs (2). High-grade narrowing of the coronary arteries due to diffuse intimal thickening and increase wall thickness of the great vessels leads to a dilated or a narrowed aorta, particularly in the isthmus, mimicking a coarctation. Due to non-metabolized GAGs predilection for deposition and failure to be scavenged, it is preferred to replace than repair the valve (6).
The body habitus of these patients is generally small, and with morphologically abnormal valves with less flexibility and poor tissue quality, smaller sized prosthetic valves are usually implanted (2). There have been 11 patients reported in the literature, of which eight patients underwent combined aortic and mitral valve replacement, one underwent mitral valve replacement, one underwent aortic valve replacement, one underwent coronary artery bypass grafting, and one underwent repair of coarctation of aorta (4).
Histopathology and electron microscopy have shown that within the cardiac valves, endocardium, myocardial walls, coronary arteries, aorta, and the conduction system are infiltrated with clear cells and granular cells with increased GAGs content. Left ventricular hypertrophy, left ventricular dilatation, left atrial and/or left ventricular volume overload, and ultimately systolic and diastolic dysfunction are the endpoints of these pathologic cardiomyopathic and valvular changes. Strategic myocardial protection is a very important key in avoiding low cardiac output or the need for mechanical circulatory support postoperatively. In addition, some patients may have respiratory issues due to macroglossia, narrowed airway, and stiff neck joints which may require fiber optic intubation by a skilled provider.
Diagnostic assessment as well as surveillance monitoring such as echocardiography, x-rays, pulmonary function test, CT scan, and CMR are important tools that may alter therapy and disease progression. Echocardiography is important in the diagnoses of valvular, systolic, and diastolic function. Hematopoietic stem cell therapy, cord blood transfusion, or enzyme replacement therapy have been widely studied, used, and monitored, but with variable outcomes. Laronidase has poor results in poorly vascularized tissues such as central nervous system, cornea (vision), articular cartilage (joint movements), and valves, but may prevent valve involvement or effectively improve diastolic and systolic function when treatment is initiated early (4, 5, 7, 8). Mucopolysaccharidosis identified at birth can be timely monitored and appropriately treated, and outcomes can be more predictable, affording better quality of life.
References
- Minakata M, Konishi Y, Matsumoto M, Miwa S. Surgical treatment for Scheie's Syndrome (Mucopolysaccharidosis Type I-S). Report of two cases. Jpn Circ J. 1998; 62: 700 –703.
- Seward JB, Casaclang-Verzosa G. Infiltrative cardiovascular diseases. J Am Coll Cardiol. 2010;55:1769-79.
- Murashita T, Kobayashi J, Shimahara Y, Toda K, Fujita T, Nakajima H. Double-valve replacement for Scheie’s Syndrome Subtype Mucopolysaccharidosis Type 1-S. Ann Thorac Surg. 2011; 92:1104 –5.
- Soliman O, Timmermans GM, Nemes A, Vletter WB, Wilson ten Cate J, Geleijnse M. Cardiac abnormalities in adults with the attenuated form of mucopolysaccharidosis type I. J Inherit Metab Dis. 2007;30:750–7.
- Braunlin E, Harmatz P, Scarpa M, Furlanetto B, Kampmann C, Loehr J, Ponder K, Roberts W, Rosenfeld H, Giugliani R. Cardiac disease in patients with mucopolysaccharidosis: presentation, diagnosis and management. J Inherit Metab Dis. 2011;34:1183–97.
- Masudo H, Morishita Y, Taira A, Kuriymu,M. Aortic Stenosis associated with Scheie’s Syndrome. Chest. 1993;103:968-70.
- Harada H, Uchiwa H, Nakamura M, Satoko Ohno S, Morita H, Atsushi Katoh A, Yoshino M, Ikeda H. Laronidase replacement therapy improves myocardial function in mucopolysaccharidosis I. Mol Gen Metab. 2011:103:215–9.
- Fischer T, Lehr H, Nixdorff U, Meyer J. Combined aortic and mitral stenosis in mucopolysaccharidosis type I-S (Ullrich-Scheie syndrome). Heart. 1999; 81:97–9.
- Butman S, Karl L, Copeland J, Combined aortic and mitral valve replacement in an adult with Scheie’s Disease. Chest 1989; 96:209-10.
- Gross DM, Williams JC, Caprioli C, Dominguez B, Howell RR. Echocardiographic abnormalities in the mucopolysaccharide storage diseases. Am J Cardiol. 1988; 61:170-6.
- Kitabayashi K, MD, Matsumiya G, Ichikawa H, Matsue H, Shimamura K, Sawa Y. Surgical treatment for mitral stenosis in Scheie’s Syndrome: Mucopolysaccharidosis Type I-S. Ann Thorac Surg. 2007;84:654 –5.