ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Thoracoscopic Right Upper Lobectomy Using a Full Posterior Dissection
Ojanguren A, Heyndrickx M, Gossot D. Thoracoscopic Right Upper Lobectomy Using a Full Posterior Dissection. November 2014. doi:10.25373/ctsnet.21365514
Introduction
Because lung cancer most often develops in the right upper lobe, right upper lobectomy is the most performed resection. However, it is not the simplest one, due to the features of its vascular and bronchial anatomy. Now that lobectomies are increasingly carried out via thoracoscopy (1), at least for early stage lung cancer, surgeons are looking for a straightforward and reproducible technique. In this context, the anterior approach and fissureless technique have gained popularity (2). However, the authors have found that this approach has some limitations and advocate for a posterior approach with gradual dissection of the fissure and of the pedicle elements from the back to the front. The authors herein report this technique, which is a full thoracoscopic one with a 100% video display, no access incision, and uses only trocars and endoscopic instruments (3).
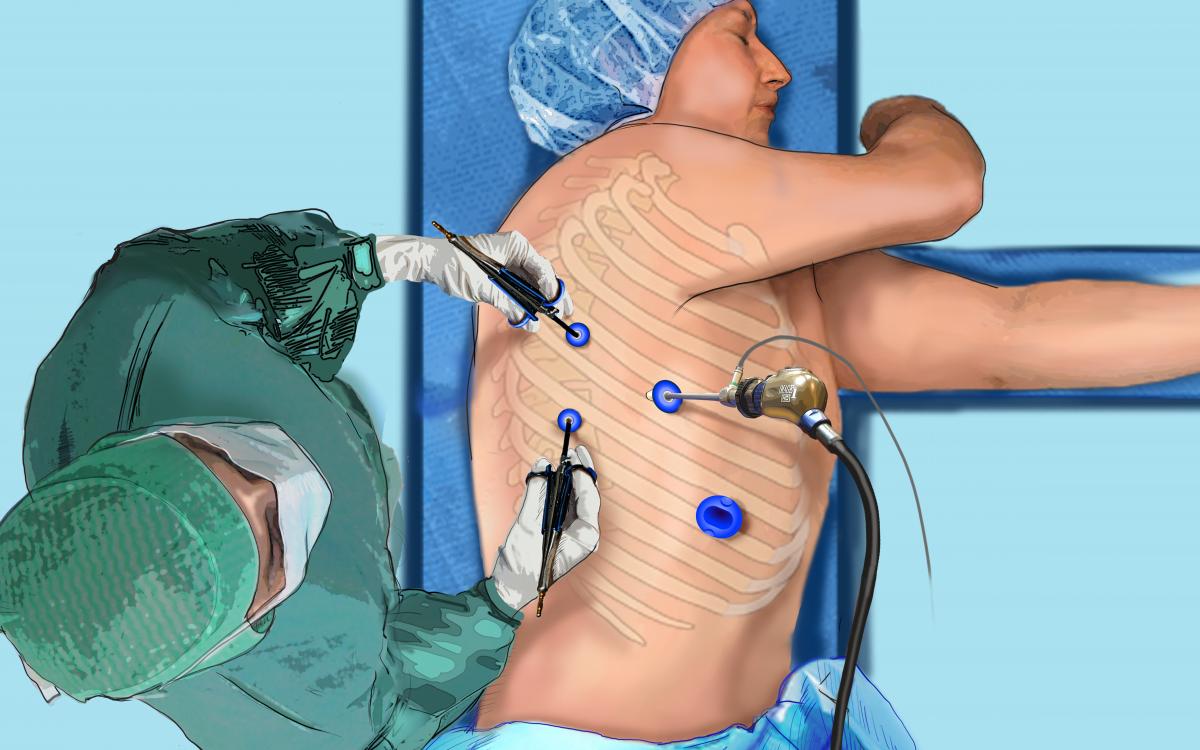
Fig.1: Position of the surgeon at the back of the patient and port placement for a thoracoscopic
right upper lobectomy
Patient Selection
Any patient selected for VATS right upper lobectomy is eligible for the posterior approach. According to ACCP recommendations, these indications are restricted to stage I tumors.
Inclusion Criteria:
- Early-stage non-small-cell lung cancer
- Tumor size < 7cm
Exclusion Criteria:
- Chest wall involvement
- Previous thoracotomy
- Hilar structures involvement
- FEV1 or DLCO < 30%
Preference Card
- Deflectable scope
- Usual range of 5 mm endoscopic instruments (grasping forceps, DeBakey forceps, small and large dissectors, scissors, suction)
- 5 mm bipolar shears
- 5 mm vessel sealing device
- 5 mm endopeanuts
- 10 mm clip applier
- Endo-stapler, preferably with curved tip
- Large retrieval bag
- Conventional thoracic instrumentation ready on a separate table
 |  |  |
| Fig. 2A: Deflectable endoscope used for full thoracoscopic approach | Fig. 2B: Example of 0° view on the right upper mediastinum | Fig. 2C: Same position of the endoscope with 90° bending. |
Operative Steps
The procedure is performed under general anesthesia with split ventilation using a double-lumen endotracheal tube. The patient is positioned in lateral decubitus, as for a thoracotomy. The surgeon stands at the back of the patient (Fig.1). Two monitors are used and the scope is held by a robotic or mechanical scope holder. The authors use a deflectable scope (LTF™, Olympus, Tokyo, Japan) housing a charge-coupled device at its tip connected to a high definition camera (Exera II™, Olympus, Tokyo, Japan) that provides sharp viewing, thus allowing for close-up vascular dissection (Fig.2). Only endoscopic instruments that have been especially designed for full thoracoscopic procedures are used (Fig.3). These are inserted through four trocars: two 5 mm trocars in the posterior axillary line for dissecting instruments, one 10 mm port for the endoscope in the middle axillary line, and one large oval shaped port in the anterior axillary line for large instruments and staplers. This port will be enlarged, if necessary, upon completion of the lobectomy for specimen retrieval (Fig.4). An additional 3 mm grasping forceps can be used, if necessary, for lung retraction. The grasping forceps are inserted directly through the skin without a trocar (Video 1). The control of large vessels is accomplished with endostaplers (Endo-GIA II, Covidien Autosuture, Mansfield, MA). Hemostasis of small-caliber vessels is performed with clips, a vessel sealing device (Enseal®, Ethicon Endo-Surgery), or a combination of both. The interlobar plane is divided with a combination of bipolar sealing device (for its peripheral thin portion), and stapling (for its central and thick portion) using 4.8 mm staples. On completion of the pulmonary resection, the specimen is placed into an endobag and retrieved through one of the port sites that was enlarged to a length of 2 to 4 cm, depending on specimen size. In most cases, only one chest tube is placed through one of the port sites. The lymph node dissection, the technique for which has been reported elsewhere (3), will not be described.
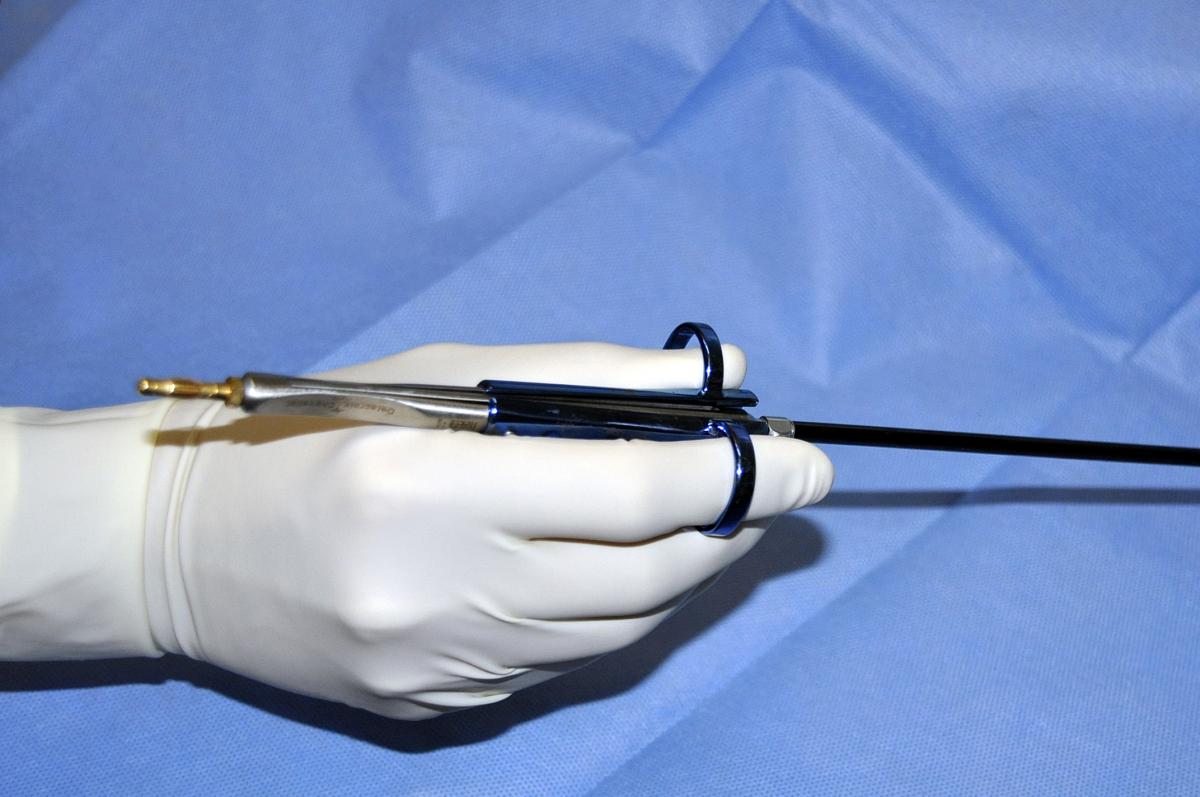 | 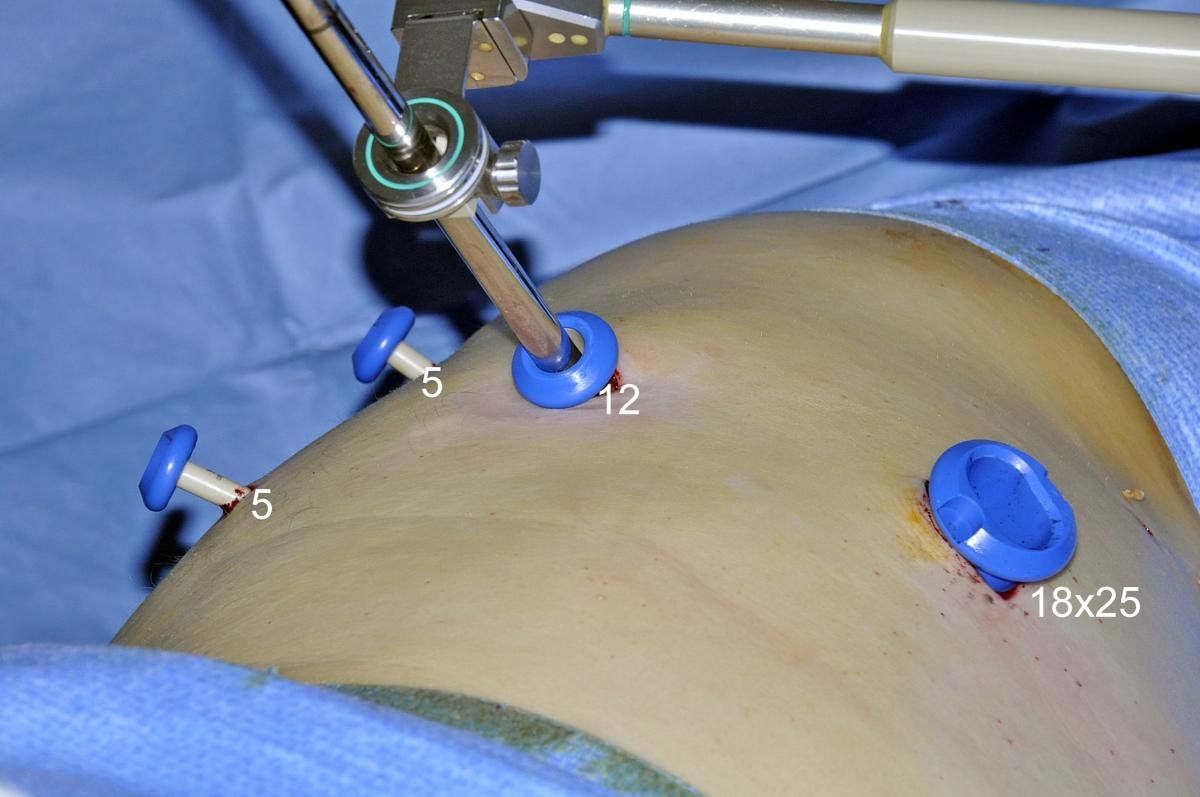 |
| Fig.3: Example of thoracoscopic instrument used during full thoracoscopic lobectomies | Fig.4: Ports |
Video 1: Use of a 3 mm grasping forceps as an additional retraction tool
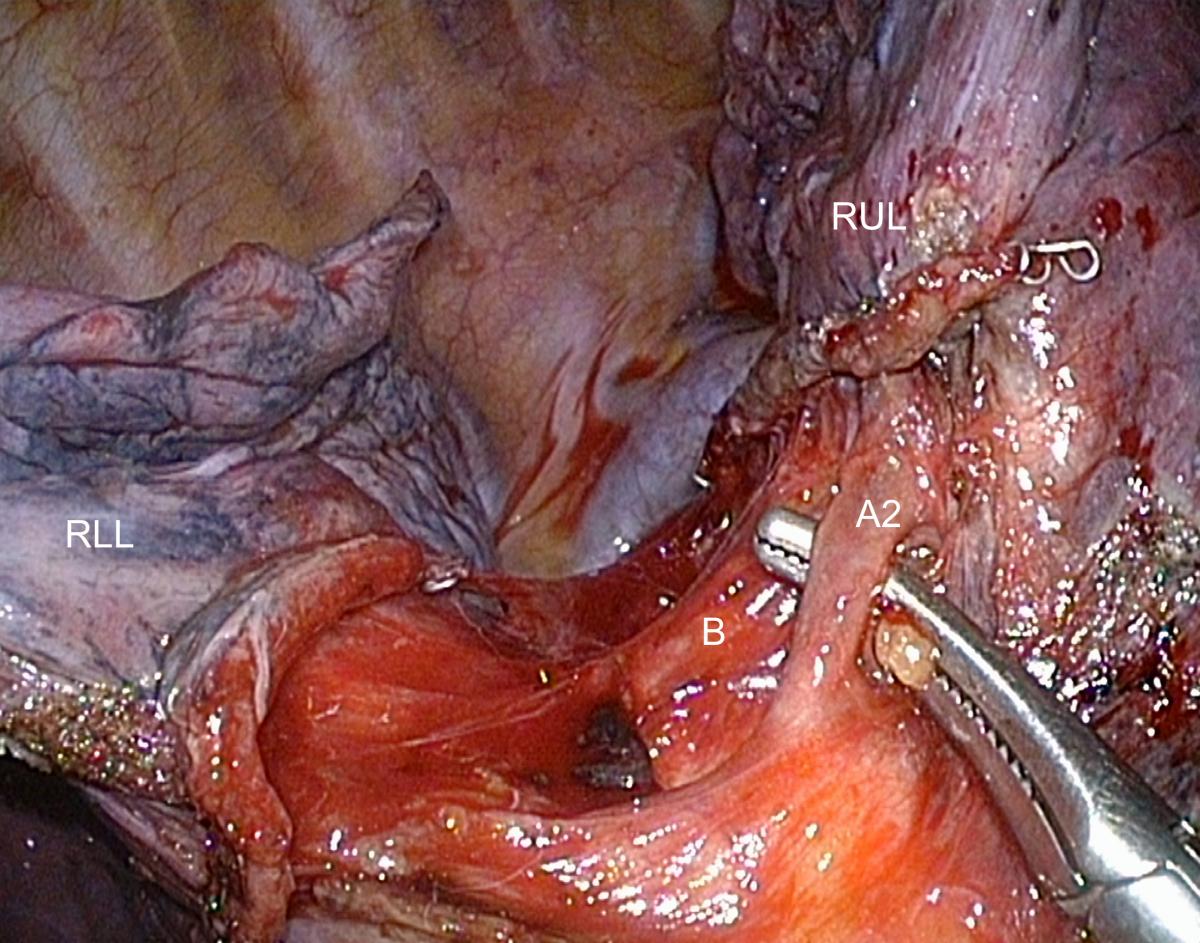
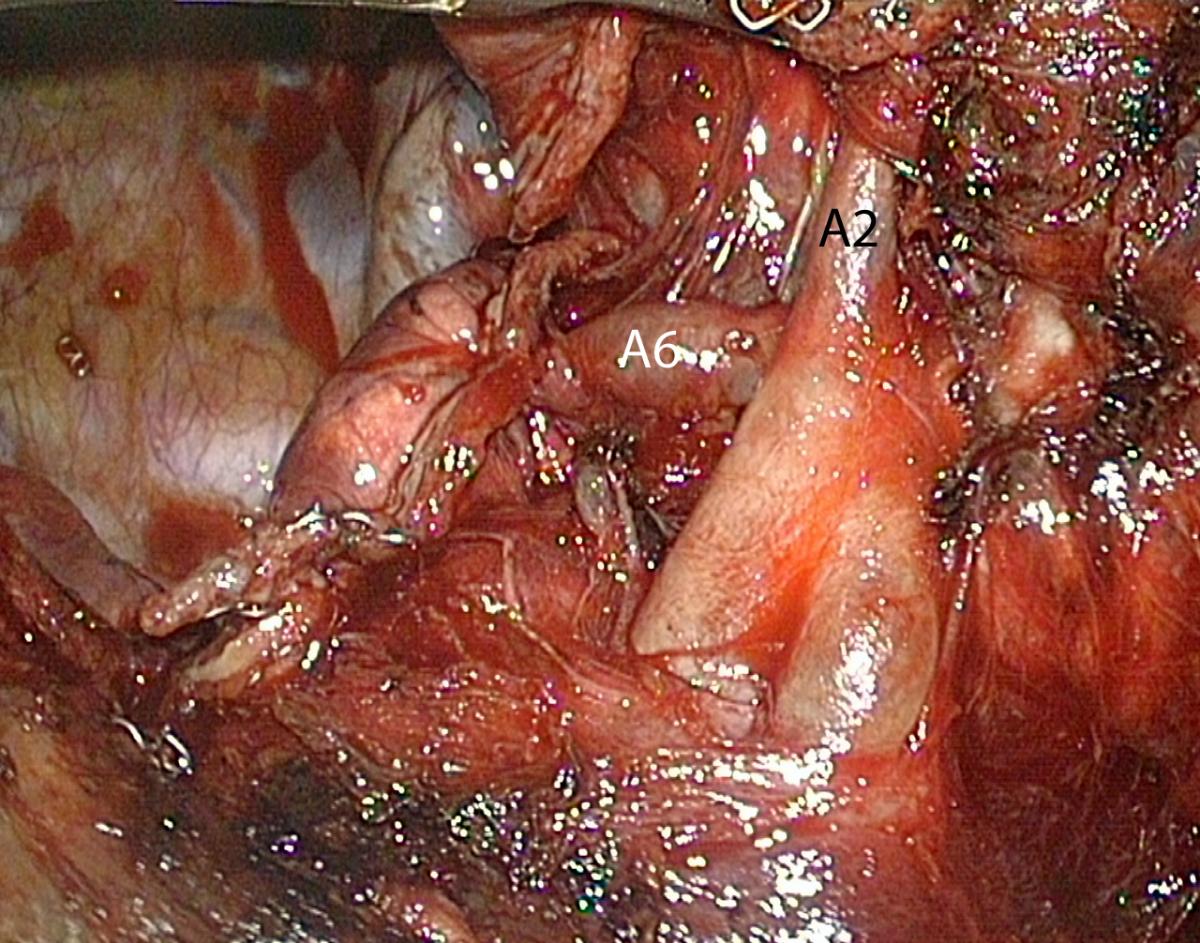
Opening of the Posterior Part of the Major Fissure and Dissection of the Posterior Ascending Artery (A2)
The first stage of the posterior approach for right upper lobectomy is the dissection of the interlobar portion of the pulmonary artery within the major fissure. This step can be tedious when the fissure is incomplete or inflammatory, but it is always feasible. Opening the fissure may lead to troublesome minor pulmonary tears and oozing. Careful dissection using a combination of bipolar diathermy and blunt dissection is required until the artery is discovered (Video 2). Once the interlobar artery is identified, the ascending branch to the upper lobe (A2) is dissected (Fig.5). In the case of a long and thick fissure, it may be preferable to first divide its posterior portion, as this will facilitate the exposure of the posterior artery (Video 3). This is achieved by stretching the upper lobe or lower lobe forward until the posterior mediastinum is fully exposed. The pleura facing the inferior aspect of the upper bronchus is opened using either cautery, blunt dissection, or both. A dissecting forceps is then passed from the hilum to the periphery under direct vision, thanks to the deflectable scope. Continuous optical control must be maintained to avoid injury of the azygos vein or the esophagus by the tip of the dissector. A curved-tip 60 mm endostapler can then be applied. This helps to expose the posterior arteries whose number ranges from one to three. The ascending arteries are clipped and divided. A vessel sealing device can also be used if the vessels are small enough.
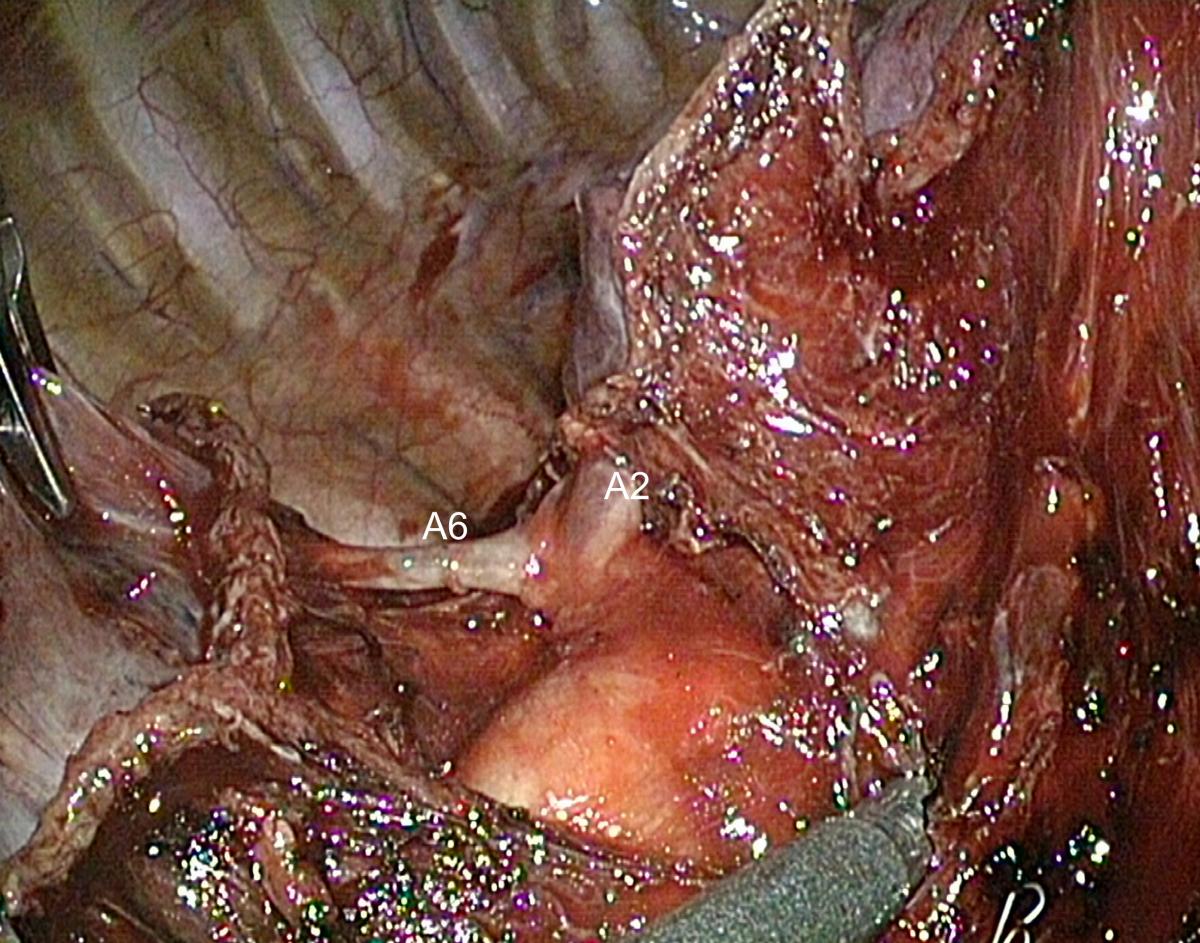
This thorough dissection of the posterior portion of the fissure helps in detecting anatomical variations such as a common birth of A2 and A6 arteries (Fig.6)
Video 2: Opening the posterior portion of the major fissure and controlling the posterior ascending artery (example of a thin fissure)
Video 3: Opening the posterior portion of the major fissure and controlling the posterior ascending artery (example of a thick fissure)
Control of the Bronchus
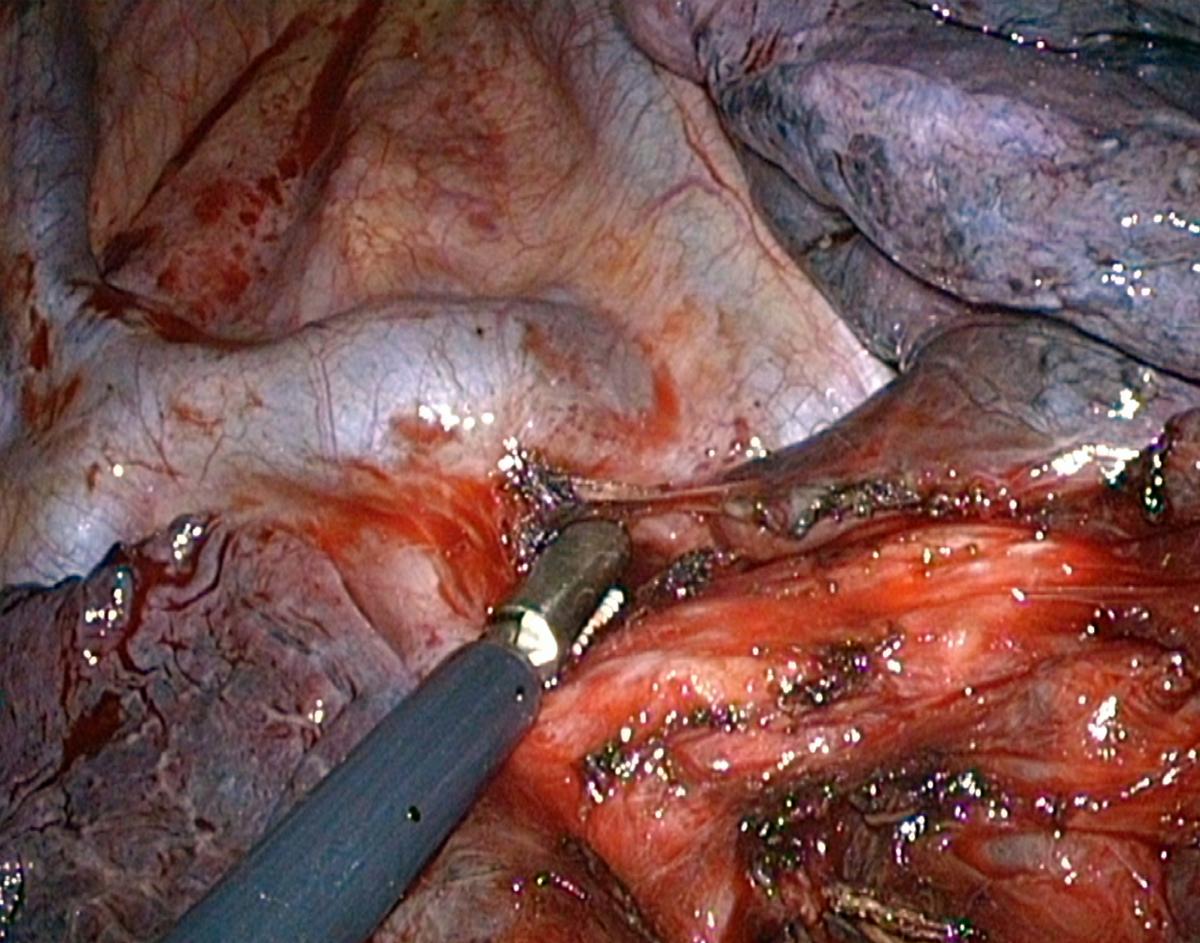
The divided A2 artery greatly facilitates the access to the upper lobe bronchus. All surrounding tissues of the anterior, inferior, and superior aspects of the bronchus are cleared. A blunt tip device or an endopeanut is then gently passed around its anterior aspect, always keeping it flush against the bronchus to avoid injury of the truncus anterior, which lies anteriorly. A sling can then be passed around the bronchus (Fig.7). It helps retracting it backward, allowing the stapler to be passed smoothly.
Fig.7: Backward retraction of the right upper lobe bronchus using a loop
Video 4: Posterior control of the right upper lobe bronchus
Control of Truncus Anterior
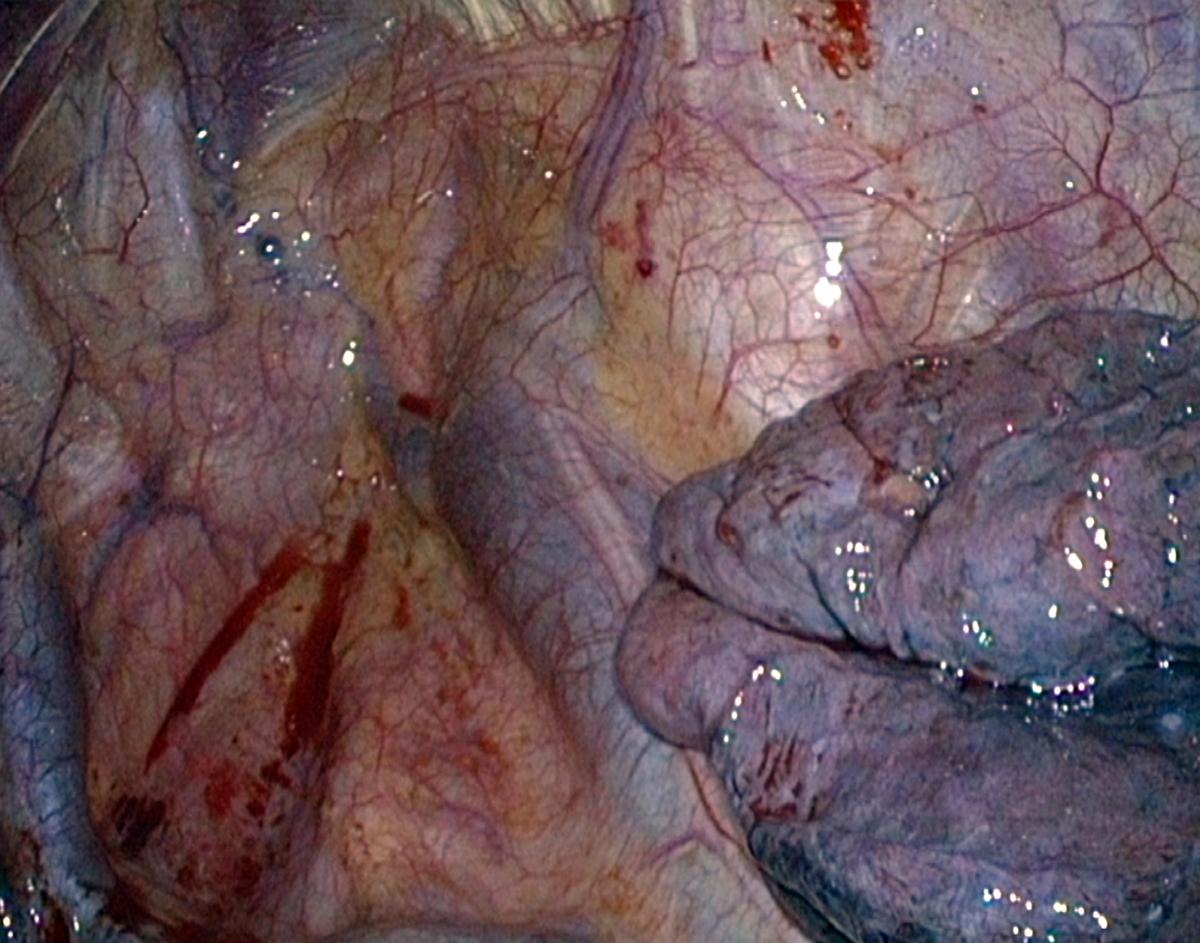
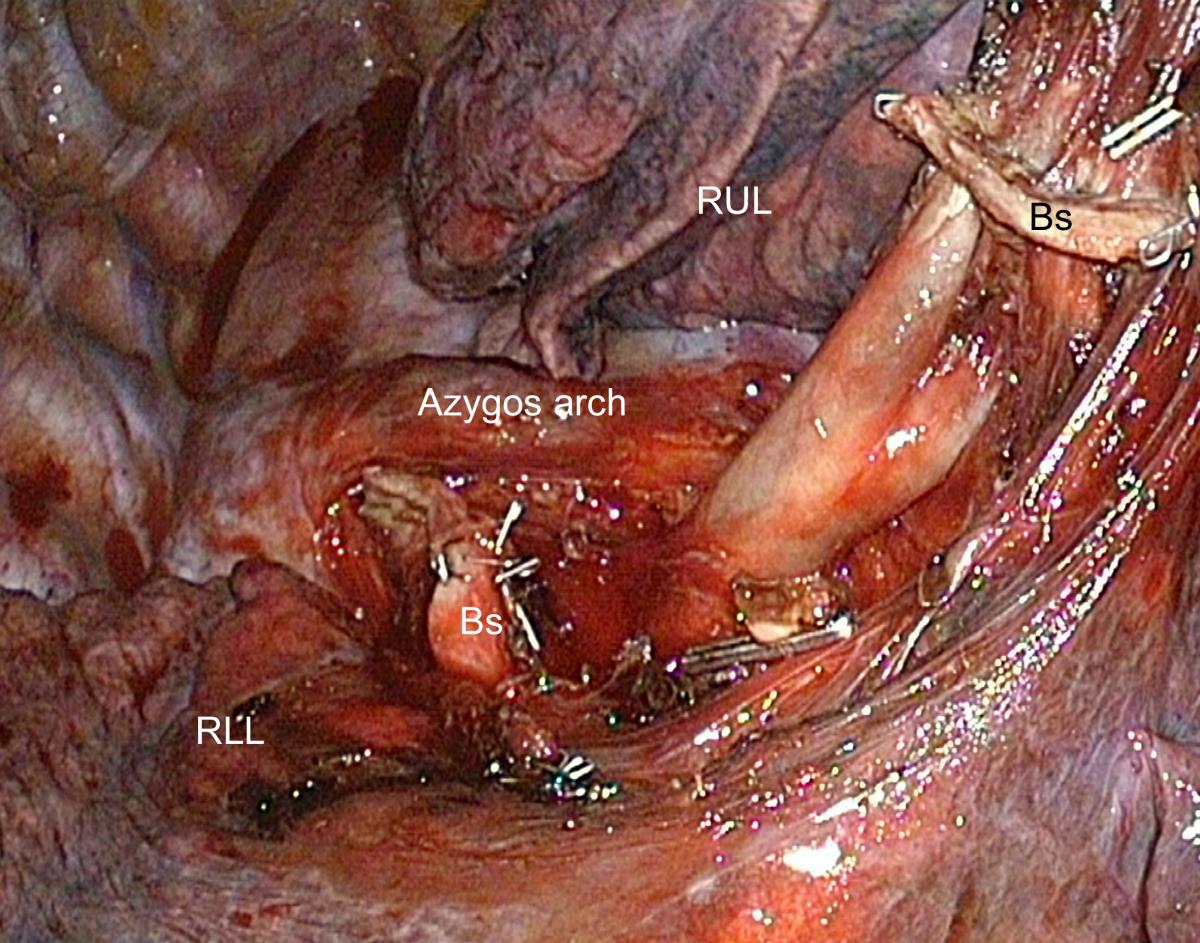
Severing the lobar bronchus exposes the two branches (A1 and A3) of the truncus anterior, which can then be dissected from behind (Fig.8). Lymph nodes are frequently seen at the level of arterial bifurcation and must be dissected and removed.
Fig.8: Exposure of the truncus anterior after division of the upper lobe bronchus
(Bs = Stump of the right upper lobe bronchus; RUL = Right upper lobe; RLL = Right lower lobe)
Video 5: Posterior control of the truncus anterior
Control of the Upper Root of the Superior Pulmonary Vein
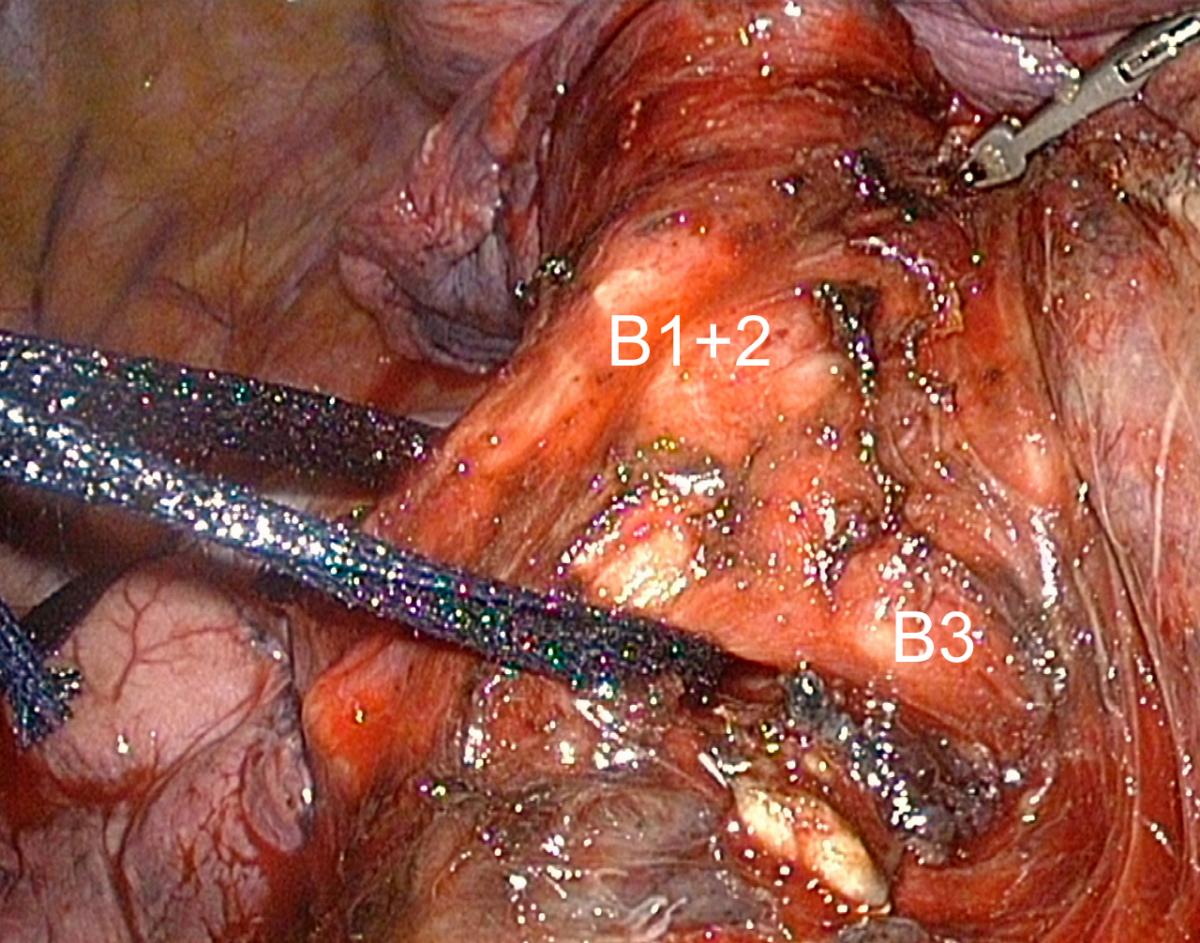
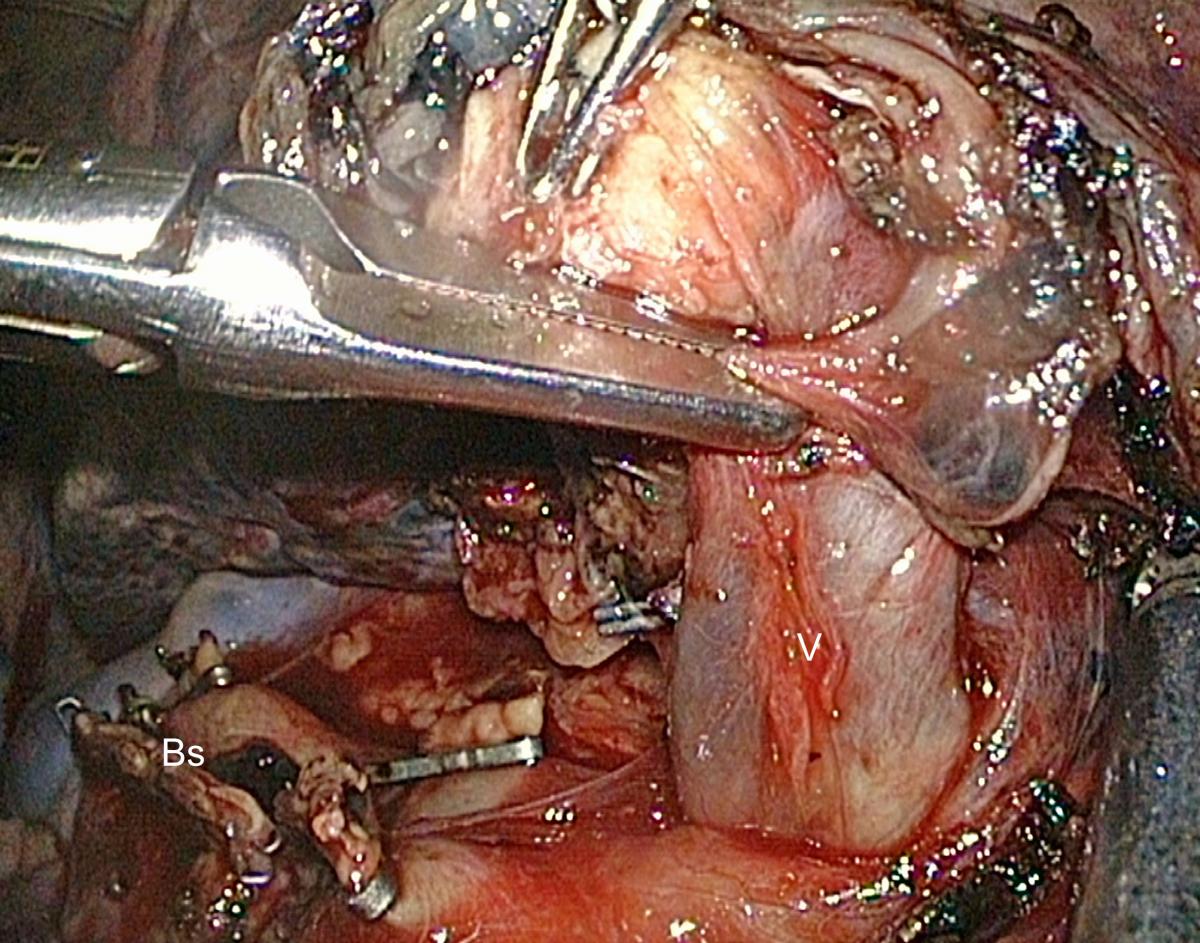
Division of the truncus anterior gives access to the upper root of the right superior vein that consists of three segmental branches: apical (V1), posterior (V2), and anterior (V3). Dissection of its posterior and lateral aspects can be done from behind by a combination of bipolar cautery and a gentle sweeping motion with endopeanut (Fig.9). The middle lobe vein is not visible from this posterior approach and does not require identification as during an anterior approach (a sometimes difficult step in some overweight patients). Should the posterior dissection of the upper root of the right superior vein be difficult, it can be completed from the front side. The upper lobe is pulled backward, the mediastinal pleura is incised posterior to the phrenic nerve down to the superior pulmonary vein, and the venous branch to the middle lobe is clearly identified.
Fig.9: Exposure of the upper root of the superior pulmonary vein.
(V = vein; Bs = Stump of the right upper lobe bronchus)
Video 6: Posterior control of the upper root of the superior pulmonary vein
Division of the Minor Fissure
The transverse fissure is rarely complete, thus the parenchyma has to be divided. After having checked that the venous branch to the middle lobe is remote, a clamp is applied on the parenchyma and its correct position is adapted according to the reventilation test. Some pressure is applied on the clamp in order to mark the parenchyma, and then the stapler is applied. One to three 4.8 mm cartridges may be necessary to complete the fissure division. The specimen is retrieved in a bag. After extraction, the pulmonary ligament is freed and the middle lobe is secured.
Video 7: Division of the minor fissure
Division of the Pulmonary Ligament
The pulmonary ligament is divided up to the inferior pulmonary vein using both diathermy and gentle traction on the lower lobe (Fig.10).
Fig.10: Division of the pulmonary ligament. Note the use of a 3 mm grasping forceps for traction on the
lower lobe.
(E= Esophagus; IVC = Inferior vena cava; D = Diaphragm)
Securing the Middle Lobe
When the minor fissure is complete, the middle lobe must be repositioned and secured to the lower lobe (Fig.11). Since this is better done under reventilation, there is not much room for hand suturing, and it is thus easier to use a stapler without knife (Endo-TA™, Covidien).
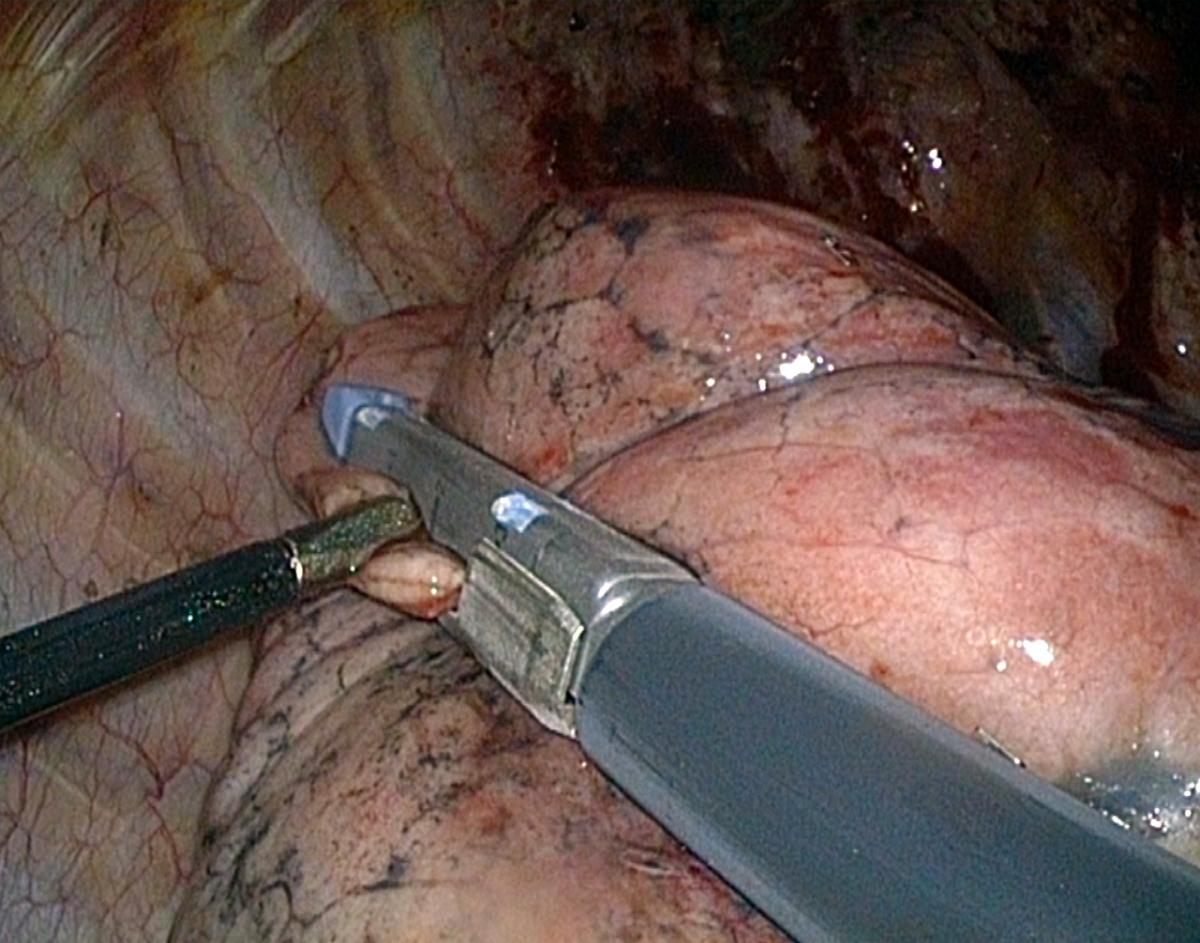 | 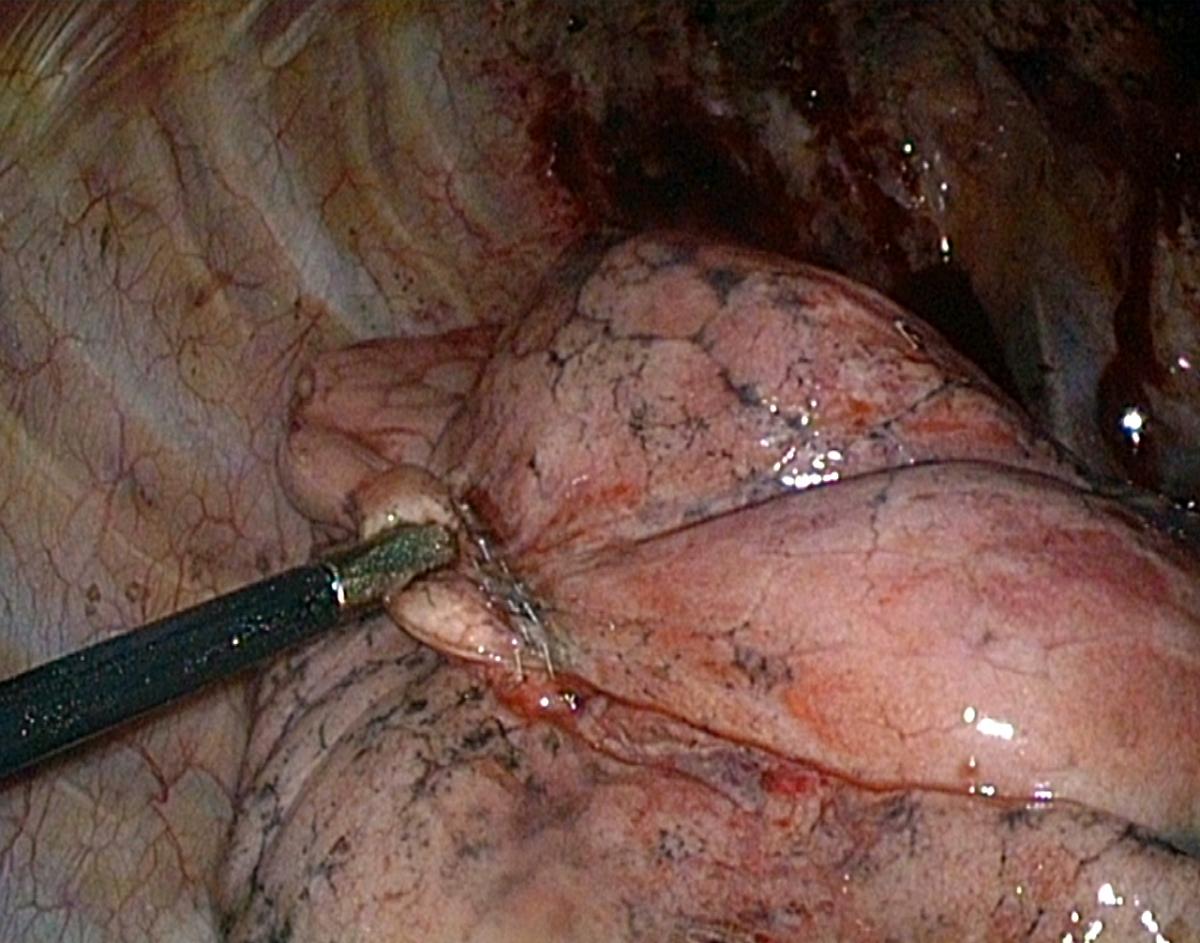 |
| Fig.11A: Securing the middle lobe by anchoring it to the lower lobe, application of an Endo-TA | Fig. 11B: Final result |
Tips and Pitfalls
- The trickiest part of the procedure is the dissection and opening of the posterior portion of the fissure, if it is large and thick. Dissection must be started at the edge of the middle lobe, with gentle opening using a combination of blunt dissection with endopeanuts and bipolar dissection.
- The posterior portion of the major fissure must not be stapled before the posterior ascending artery has been distinguished.
- Posterior dissection of the upper lobar bronchus is easy and safe. But, if adherent lymph nodes are present, there is a risk of tearing the truncus anterior that runs just in front of the bronchus. In this case, one has to convert to an anterior or a combination of posterior and anterior dissection.
- Maintaining optimal vision of the whole operative field is a major concern as an instrument tip may be out of vision. To overcome this problem, a deflectable scope with an angle of vision from 0º to 100º allows the surgeon to have a bird’s eye view, making dissection more natural and safer.
- To avoid conflicts with the instruments or the hands of the assistant, a mechanical telescope holder is used. It is placed at the front of the patient at the level of the scapula tip for the posterior approach of right pulmonary resections, thus avoiding conflict with instruments shafts.
- Exposure of the hilum or fissure may be facilitated using an additional 3 mm grasping forceps for lung retraction. It is inserted directly through the skin without a trocar, thus reducing trauma to minimum.
Comments
The anterior approach, which is the most commonly used for thoracoscopic lobectomies, has some advantages: it is rather simple, mimics the anterior dissection of the truncus anterior and of the upper vein as done via open surgery, and eliminates the need to dissect the fissure, if the latter is too difficult to open and dissect (2). This is known as fissureless technique. It has, however, some drawbacks:
- In some patients, especially in obese ones, the dissection of the truncus anterior and of the upper vein can be dangerous, as these two structures can be difficult to identify if surrounded by fat. In addition, there is a risk of confusion and anatomic misjudgment of the two structures because of their proximity (5).
- Completing this approach with division of the fissure without dissection of pulmonary artery can lead to accidental tearing or stapling of an A6 artery when the latter has a common birth with A2 or even arises from A2.
The posterior approach, described and advocated by W. Walker, may seem more challenging (6). Indeed, the dissection and opening of the major fissure can be tedious if thick and inflammatory. But, the posterior approach is always feasible, it just requires patience. This allows for a safe dissection of the A2 artery and then of all elements of the pedicle. It has been our preferred route for years. For those surgeons who do prefer the anterior approach, mastering this posterior dissection can help combining different views when the anterior dissection becomes unsafe.
References
- Howington J, Blum M, Chang A, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd edition. American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(Suppl): 278–313.
- Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc. 2011;25:1263-9.
- Gossot D. Atlas of endoscopic major pulmonary resections. Paris: Springer-Verlag; 2010.
- Ramos R, Girard P, Masuet C, Validire P, Gossot D. Mediastinal lymph node dissection in early-stage non-small cell lung carcinoma: totally thoracoscopic vs thoracotomy. 2012. 2012;41:1342-8.
- Fournel L, Zaimi R, Grigoroiu M, Stern JB, Gossot D. Totally thoracoscopic major pulmonary resections: an analysis of perioperative complications. Ann Thorac Surg. 2014;97:419-24.
- Richards J, Dunning J, Oparka J, Carnochan F, Walker W. Video-assisted thoracoscopic lobectomy: the Edinburg posterior approach. Ann Cardiothoracic Surg 2012;1:61-9.
Disclaimer
The information and views presented on CTSNet.org represent the views of the authors and contributors of the material and not of CTSNet. Please review our full disclaimer page here.






Comments