ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Treatment of left ventricular pseudoaneurysm through left thoracotomy on a ‘beating’ heart
Index
INDEX
Introduction
Left ventricular pseudoaneurysms represent a rare condition where cardiac rupture is contained by adherent pericardium or scar tissue. They most commonly occur after myocardial infarction (MI). We present here a case of a 78 year-old male with history of coronary artery revascularization who was found with a large pseudoaneurysm of the posterior wall of the left ventricle. Coronary angiogram showed patency of all coronary grafts and preserved ventricular function. The clinical challenge of treating such a complex condition was amplified by the need to preserve the previous by-passes and by the challenge of exposing safely the posterior left ventricle in the face of a redo-sternotomy. Left antero-lateral thoracotomy offered the advantage of a virgin surgical field where approach to the posterior left ventricle could be accomplished minimizing the dissection around the heart and avoiding the risk of damaging the previous coronary bypasses. The issue of myocardial protection was solved by maintaining the heart beating. Alternative surgical approaches and “beating” heart surgery can be a useful tool in the armamentarium of a cardiac surgeon to treat complex pathologies.
 |
 |
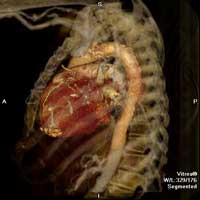
| Figure 1 & 2. Preoperative CT-scan | |
Case Report
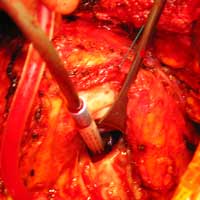 |
| Figure 3. The pseudoaneurysm is opened |
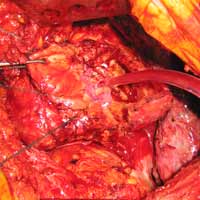 |
| Figure 4. Patch closure of the defect |
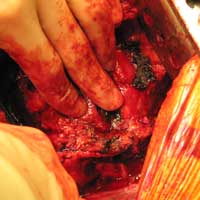
We report a case of a 78 y/o male with history of CAD, for which he underwent coronary artery by-pass surgery in 2004. The patient was admitted to the hospital with symptoms of moderate congestive heart failure. EKG showed no signs of acute ischemia and cardiac enzymes were negative. Cardiac ECHO showed a 6.8 x 5 cm pseudoaneurysm of the posterior left ventricle, well preserved ventricular function and no abnormalities of the mitral and aortic valves. Coronary angiogram demonstrated patency of all previous bypasses. The chest CT allowed better definition of the anatomy of the condition (Figure 1, 2). The procedure was performed through an antero-lateral left thoracotomy positioning the patient in a right lateral decubitus. The chest was entered at the 5th intercostal space and the sterno-costal junction was interrupted to optimize exposure. The left lung was displaced posteriorly and the pericardium was opened longitudinally and posteriorly to the phrenic nerve. Dissection of the heart surface from the pericardium was easily completed obtaining perfect exposure of the left ventricular pseudo-aneurysm. The previous by-pass grafts were never visualized, being distant from the operative field. Cardiopulmonary bypass was established through the left femoral vessels using a 17 F arterial femoral cannula and a 25 F multistage venous femoral cannula. The left ventricle was vented through the apex using a 10 F suction catheter. Systemic pressure was kept above 70 mmHg and the heart was decompressed before entering the left ventricular cavity via a longitudinal incision through the wall of the pseudoaneurysm (Figure 3). A bovine patch pericardial repair was completed with interrupted pledgetted sutures maintaining the architecture of the left ventricle (Figure 4). The closure was reinforced overlapping the wall of the pseudoaneurysm over the patch closure (Figure 5). Meticulous de-airing was completed under TTE monitoring before allowing ejection of the left ventricle into the circulation. De-airing was accomplished placing the patient in steep Trendelemburg position and maintaining full suction on the apical vent. Cardioplulmonary bypass was weaned-off without complications. The patient postoperative course was uncomplicated and he was discharged home in postoperative day 6. The postoperative CT-scan image is shown (Figure 6).
 |
 |
| Figure 5. Overlapping of the patch repair with the wall of the pseudoaneurysm | Figure 6. Postoperative CT-scan |
Discussion
Ventricular pseudoaneurysms are expressions of a perforation of the heart chamber where free rupture is contained by adhesions with the pericardium and formation of fibrous tissue (1, 2). Usually they occur after a myocardial infarction but they can also represent a complication of previous cardiac surgery, more commonly after mitral valve replacement. In the acute form, when diagnosed within two weeks from the event which caused them, they are extremely unstable and prone to fatal rupture. Chronic pseudoaneurysms are those identified more than three months after myocardial infarction or cardiac surgery and they have an imperfectly known natural history. In a review of literature, Frances and colleagues (1) found that myocardial infarction was the most common cause, followed by cardiac surgery, trauma and infection. The most common location was the inferior wall of the left ventricle and posterior infarcts accounted for approximately twice as many cases as anterior infarctions. They most commonly present with heart failure symptoms, chest pain and dyspnea, but they are asymptomatic in 10% of the cases. Diagnosis is usually made on angiography or transthoracic echo (TTE). The presence of a narrow orifice leading into a saccular aneurysm distinguishes pseudoaneurysms from true aneurysms. CT-Scan and MRI may provide further useful imaging. Most authors favor surgical intervention (2, 3), since untreated pseudoaneurysms have a 30 to 45 % risk of rupture (2-4). The surgical correction is a high risk procedure with a reported mortality above 20% (1). In the specific case that we are presenting, the patient had a previous bypass surgery with patent grafts. For this reason, the left thoracotomy approach was used to expose the pseudoaneurysm through a virgin field. The “beating” heart technique allowed us to avoid cardioplegic arrest. The use of “beating” heart surgery for the treatment of this kind of pathologies is well described in the literature (5). In our opinion, this may represent the preferred approach for treatment of ventricular pseudoaneurysms involving the inferior wall of the heart in cases where the patient already had coronary by-pass or valve surgery because prevents the risks related to the redo sternotomy and allows to approach the inferior wall of the heart through a virgin field. The repair of the defect in the ventricular wall was easily accomplished using a pericardial patch sutured in place with interrupted pledgetted sutures. In case of chronic pseusoaneurysms, such as the one described, the edge of the defect is made of scarring tissue which holds very well the anchoring of the pericardial patch even with a beating heart condition. With this technique the architecture of the left ventricle is preserved and less tension is applied to the suture line compared to a linear closure. According to the technique described by Salerno and co-authors (6), the beating heart technique was accomplished maintaining continuous venting of the left ventricle at all time and keeping systemic perfusion pressure above 70 mmHg to prevent air embolism. Before weaning-off cardiopulmonary by-pass, de-airing maneuvers are performed keeping the heart completely decompressed with steep Trendelemburg position and full suction through a left ventricular apical vent. Ejection of the heart in the systemic circulation is prevented until complete de-airing of the cardiac chambers is demonstrated on Trans Esophageal Echo (TEE).
In summary, the combination of an alternative surgical approach and technique, such as left thoracotomy and “beating” heart may be the preferred method to treat complications of myocardial infarction occurring in patients with previous coronary artery or valve surgeries. The thoracotomy approach allows easy exposure of the posterior wall of the heart through a virgin field. The beating heart technique offers the most physiologic method of myocardial protection maintaining warm blood perfusion of a decompressed beating heart.
References
- Frances C, Romero A, Grady D. Left Ventricular Pseudoaneurysm. J Am Coll Cardiol 1998;32:557-61
- Treasure T. False aneurysm of the left ventricle. Heart 1998;80:7-8
- Yakierevitch V, Vidne B, Melamed R, Levy MJ. False aneurysm of the left ventricle. Surgical treatment. J Thorac Cardiovasc Surg 1978:76:556-8.
- Vlodaver Z, Coe JI, Edwards JE. True and false left ventricular aneurysms. Propensity for the latter to rupture. Circulation 1975;51:567-72.
- Van Tassel RA, Edwrds JE. Rupture of heart complicating myocardial infarction. Analysis of 40 cases including nine examples of left ventricular false aneurysms. Chest 1972;61:104-16
- Ono M, Wolf RK. Left ventricular pseudoaneurysm late after mitral valve replacement. Ann Thorac Surg. 2002;73:1303–5. doi: 10.1016/S0003-4975(01)03268-4.
- Salerno TA, Panos AL, Tian G, Deslauriers R, Calcaterra D, Ricci M. Surgery for cardiac valves and aortic root without cardioplegic arrest (“Beating Heart”): experience with a new method of myocardial perfusion. J Card Surg 2007;22:459 464.




