ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Thoracoscopic Right Apicoposterior Segmentectomy
Gossot, Dominique; Sequin-Givelet, Agathe; Grigoroiu, Madalina; Brian, Emmanuel; Traibi, Akram (2017): Thoracoscopic Right Apicoposterior Segmentectomy.
CTSNet, Inc. https://doi.org/10.25373/ctsnet.5566726
Retrieved: 19:43, Nov 15, 2017 (GMT)
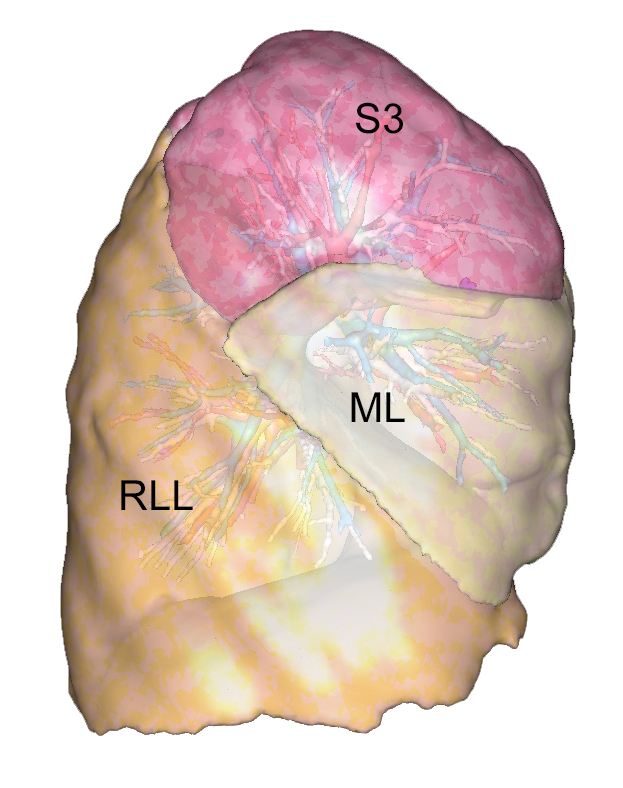
S2 and S1+2 segmentectomies are frequently indicated, as the location of a tumor or a nodule in S2 or at the border between S2 and S1 is not unusual. Preserving S3 rather than performing an upper lobectomy has two advantages: it spares respiratory function, and S3 is a large segment that occupies the pleural cavity and prevents reexpansion issues that can be encountered after an upper lobectomy, as illustrated in Video 1 and Figure 1.
The technique the authors describe is based on a thoracoscopic fissure-first approach whose rational and basics have been reported (1, 2). This technical description is based on an experience of 63 right S1+2 segmentectomies out of a series of 375 thoracoscopic segmentectomies that have been performed at the authors' institution. Knowledge of anatomical landmarks and anatomical variations is a prerequisite to feeling secure during these procedures (3).
Patient Selection
Indications are those of any other anatomical segmentectomy (4):
- Single metastasis that is too large and/or too deeply located to be suitable for a wedge resection.
- Ground glass opacities (5).
- Some carcinoid tumors.
- Some stage cT1aN0 non-small cell lung carcinomas (NSCLC) in patients for whom sparing lung parenchyma is worthwhile (6).
In this last case, segmentectomy is completed by an intersegmental and mediastinal lymph node dissection (Video 2), an approach whose survival benefit after segmentectomy was recently confirmed (7). Intraoperative examination is performed both on intersegmental lymph nodes and staple lines to decide whether segmentectomy is oncologically sufficient or whether it should be transformed into a lobectomy.
Anatomical Landmarks
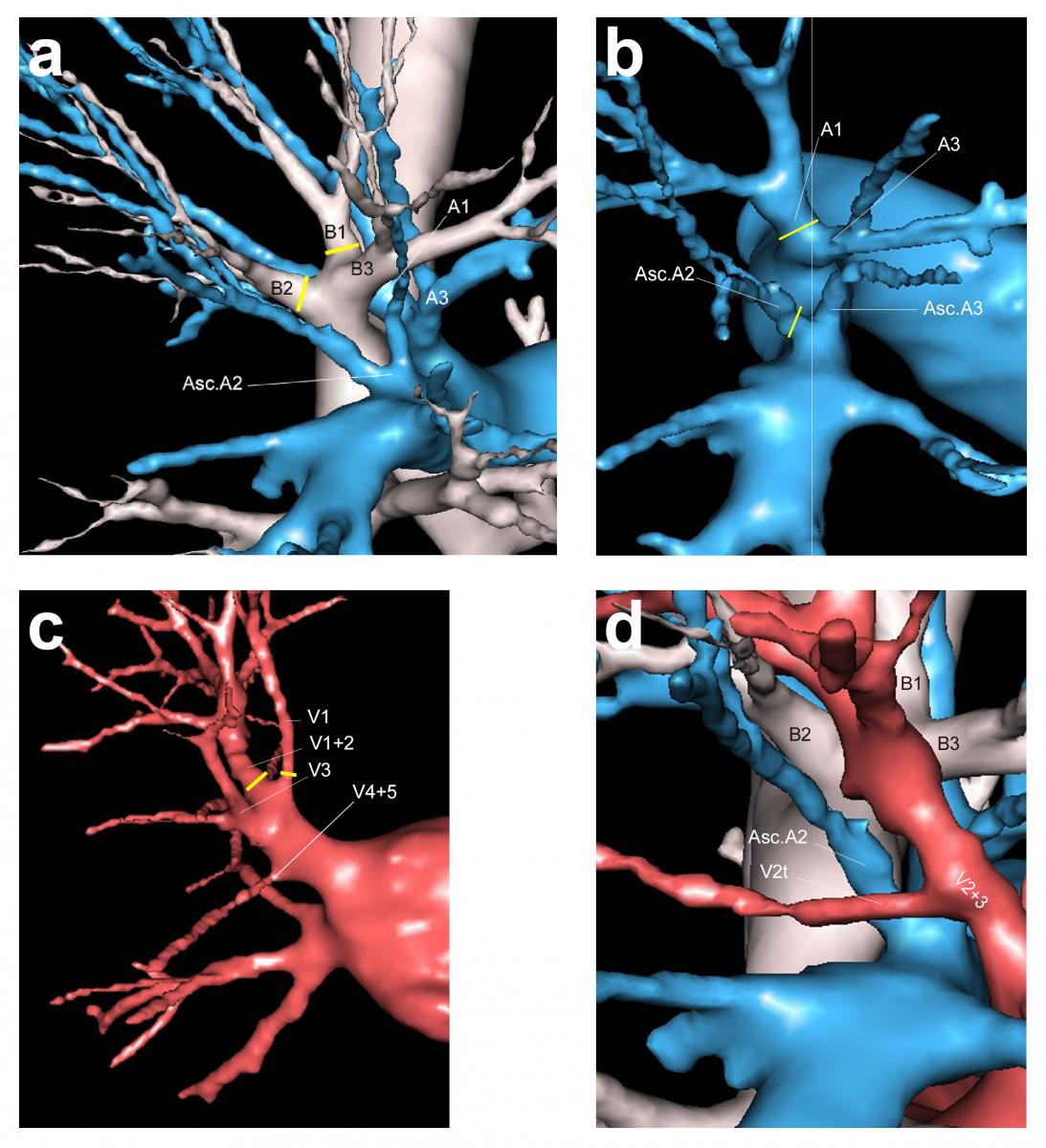
Figure 2. Anatomical landmarks. a) Segmental bronchi and arteries, posterior view. b) Arterial supply to the apicoposterior segments, posterior view. c) Venous drainage, anterior view. d) V2t crossing the posterior aspect of ascending A2, view from above.
Bronchus: As it enters the parenchyma, the upper lobe bronchus divides into three segmental bronchi: apical (B1), posterior (B2), and anterior (B3). B1 and B2 can originate separately or as a common trunk (B1+2) (Figure 2a).
Arteries: The arteries to the apical and posterior segments must be divided. They arise from the truncus anterior and from the pulmonary artery (PA) in the fissure (ascending arteries). The truncus anterior divides into 2 branches: the apical artery A1 and the anterior artery A3 that must be preserved. The posterior segment is supplied by the ascending A2 (Asc A2) that originates within the fissure from the posterior aspect of the pulmonary artery, opposite the middle lobe artery. It ascends to S2 and lies posterior to the lobar bronchus. In most patients there is only one artery, while there are none or two in some patients (Figure 2b). In some patients, a branch of A1 supplies S2 and is named recurrent A2 (Rec A2). It usually runs along the bronchus.
Veins: In most cases, the segmental veins of the upper lobe are the two upper tributaries of the upper lobe vein. V1 is the uppermost branch and is found in the hilum of the upper lobe. It is the most anterior and superior vessel. V2+3 is the central vein, and it runs in the parenchyma and within the fissure. It gives a large posterior branch for V2 and small tributaries for V3 (Figure 2c).
Operative Steps
Since 2015, most patients at the authors' institution have preoperative 3-dimensional (3D) modeling using the CT scan with intravenous contrast. The interest and benefit of 3D reconstructions for thoracoscopic segmentectomies have now been widely reported (8-10).
The authors use a full posterior approach, based on the dissection of the fissure, as described for right upper lobectomies.
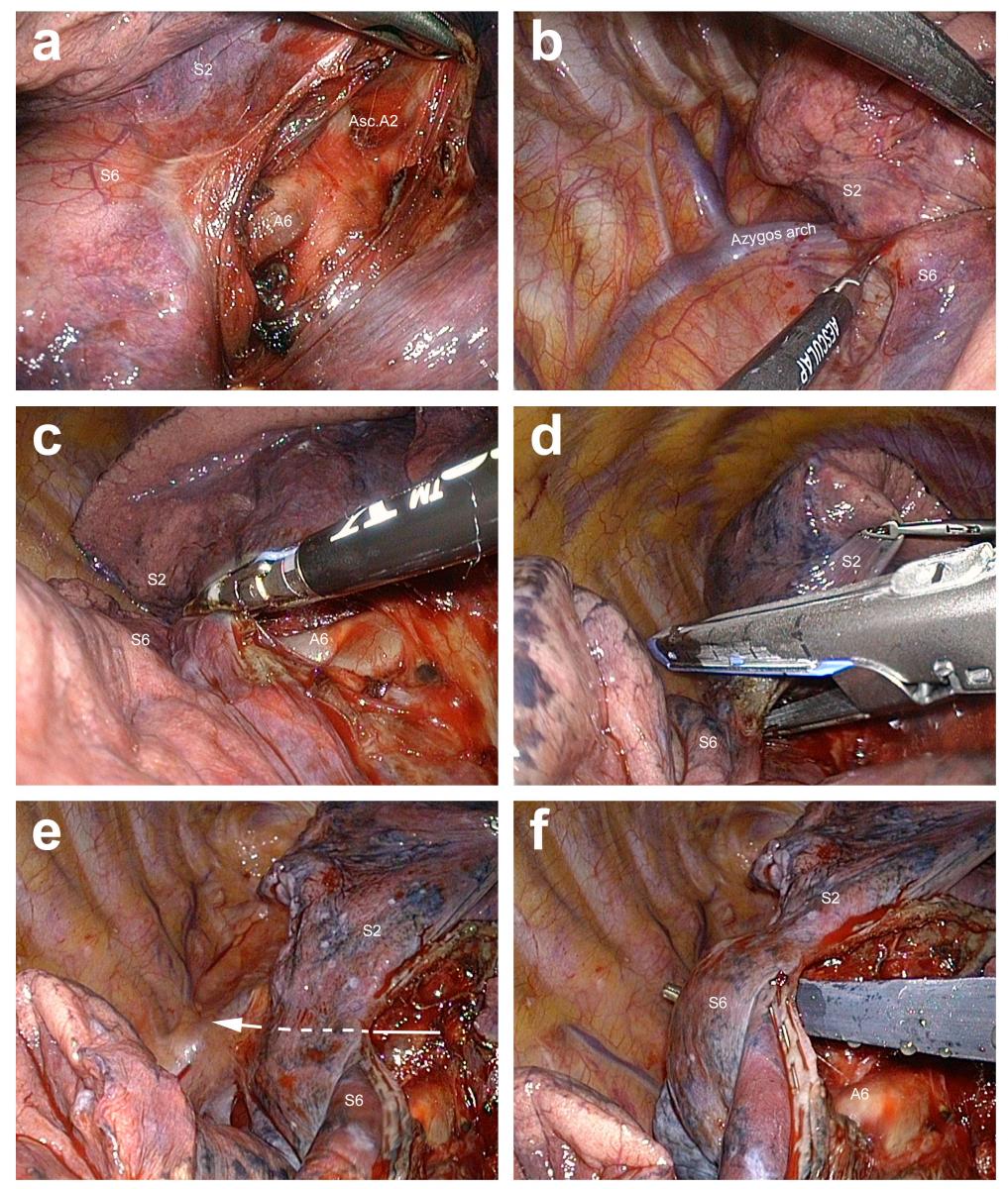
Figure 3. Division of the posterior part of the oblique fissure. a) Opening the fissure in front of the pulmonary artery, until both A2 and A6 are identified. b) S2 and S6 are pulled forward to expose the posterior mediastinum. The mediastinal pleura is opened. c) Fissure division is initiated with a vessel-sealing device. d) When long, the fissure is made shorter by stapling. e) The direction of the tunnel is now clear and denoted by the arrow. f) A blunt tip instrument can be passed in the tunnel to prepare the insertion of a stapler.
Fissure and Ascending Posterior Artery
It is more convenient to start the resection by controlling and dividing the bronchi. However, this can be done only if the ascending posterior artery, which hides the upper lobe bronchus, is severed. The posterior aspect of the oblique fissure is opened as described for right upper lobectomies (Figure 3).
This is achieved by retracting the upper lobe or lower lobe anteriorly, until the posterior mediastinum is fully exposed. The pleura facing the inferior aspect of the upper bronchus is opened using cautery, blunt dissection, or both. A dissecting forceps is then passed from the hilum—at the level of the posterior aspect of the PA—to the periphery under vision control, thanks to the deflectable scope. A 60 mm endostapler is applied. This helps exposing the ascending A2, which is then dissected and clipped or severed after bipolar sealing.
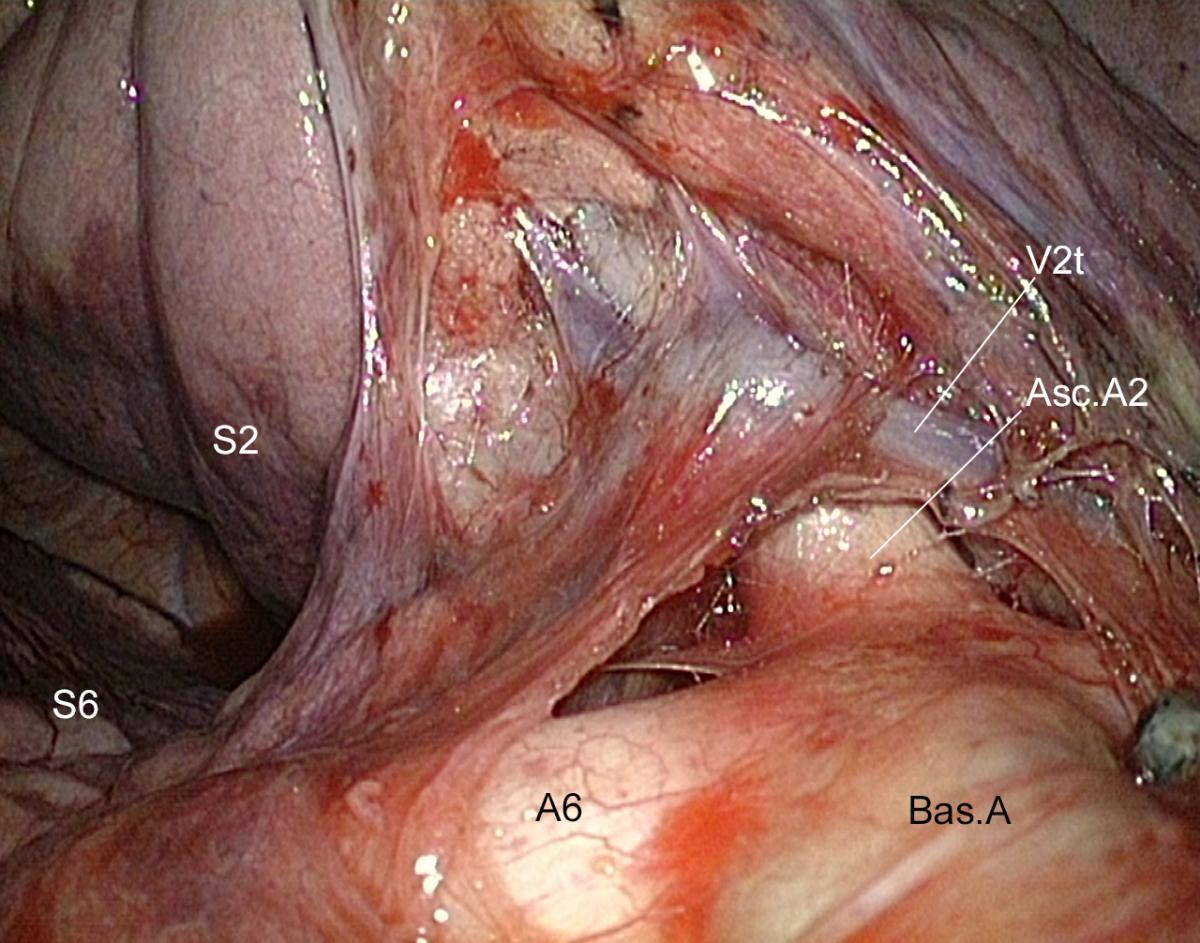
A V2t must sometimes be controlled before dissecting the ascending A2 (Figure 4). Veins can also hamper the approach to this artery (Figure 5).
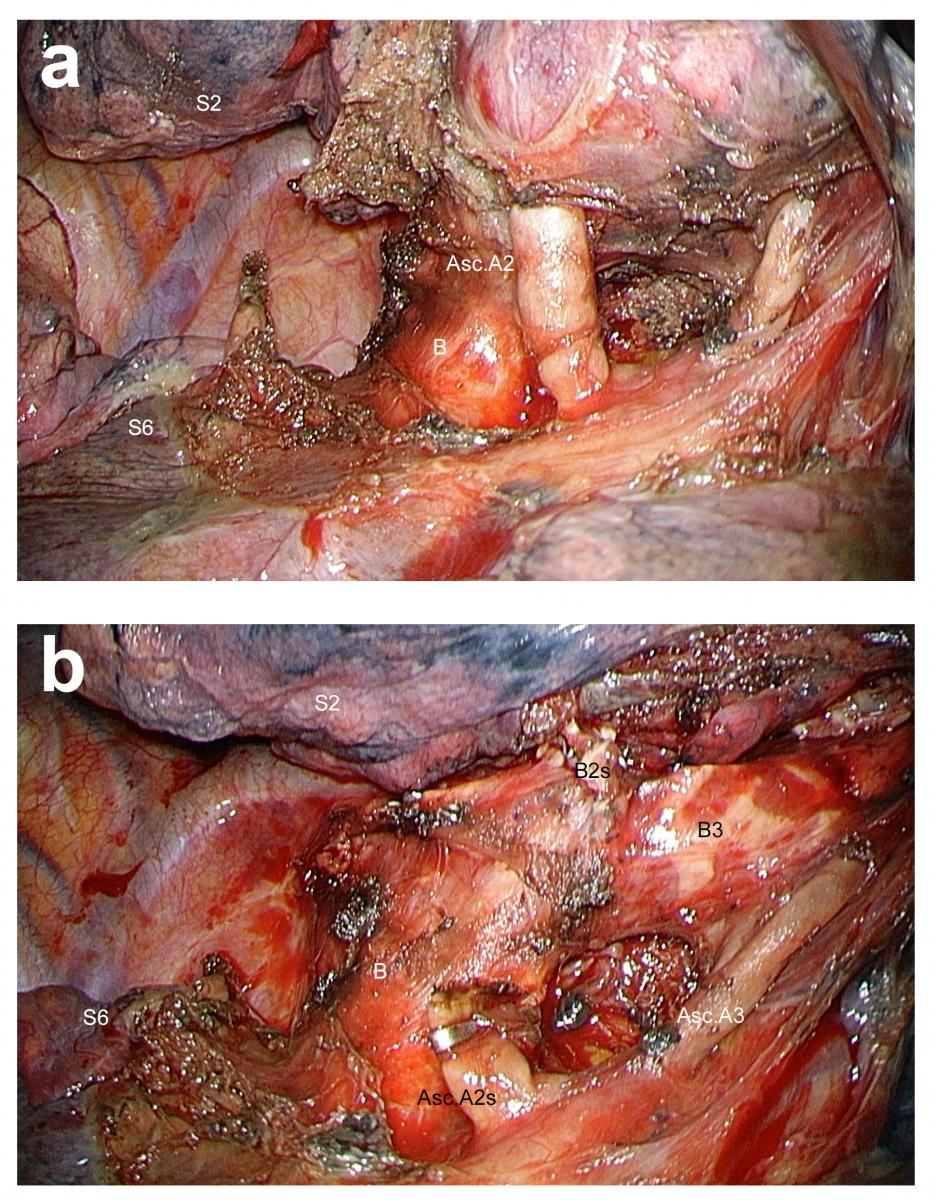
Figure 6. Presence of two ascending arteries within the fissure. a) The most anterior artery could be a second ascending A2 or an ascending A3. b) As dissection is continued, it becomes clear that this artery is for S3.
Whenever possible at this stage these veins should be pushed away rather than clipped to prevent venous congestion of the upper lobe, although this seldom occurs.
When two ascending arteries are found in the fissure, this can be a double ascending A2 or an ascending A2 (posteriorly) with an ascending A3 (anteriorly). If the distribution of these two arteries is unclear, only the posterior is divided. By pursuing dissection, the direction of the second ascending artery will become more apparent (Figure 6 and Video 4).

Figure 7. Exposure of the segmental bronchi of the right upper lobe. a) Common B1+2. b) Independent bronchi.
Segmental Bronchi
Once the ascending posterior arterial branch has been divided, the anterior surface of the right upper bronchus is fully exposed. Tissues overlying its superior aspect are freed using bipolar cautery. Clearing of the upper lobe bronchus must be pursued forward and upward until B3 becomes visible.
Dissection of the segmental bronchi is continued by a combination of gentle traction of the upper lobe and blunt dissection. This is best achieved by an endopeanut while any oozing or minor bleeding from small peribronchial arteries is immediately controlled. Finally, the trifurcation is exposed: the anterior segmental bronchus is the lowest branch, and the posterior and apical bronchi can take off independently or as a common stem (Figure 7).
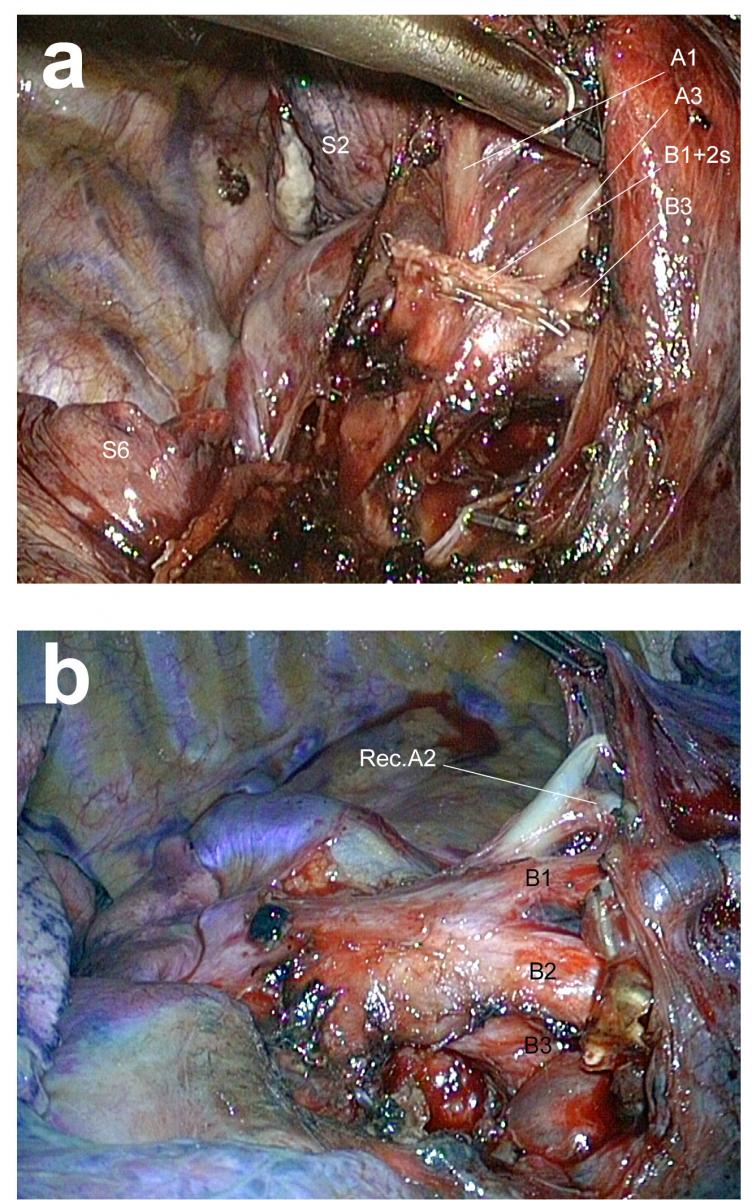
Figure 8. Control of segmental arteries. a) A1 is found in front of the B1 stump. b) Example of a recurrent A2. This maneuver liberates the apicoposterior segments, which unfold forward, thus exposing A1, which is the uppermost tributary of the truncus anterior as shown in panel a. It is dissected and stapled.
In difficult situations (fibrosis, adherent lymph nodes, etc), recognizing the anatomy of the segmental bronchi can be difficult, especially without help from preoperative 3D modeling. When it is unclear whether the first visible bronchus is B1+2 or only B2, there is no risk to first staple this bronchus, pursue dissection, and check if B1 is more deeply and anteriorly located, as in Figure 7b and Video 5.
Arteries
A1 must not be mistaken with an A1-A2 common trunk. This underscores the need for exposing all arteries. In about 10% of patients, a recurrent A2 is found running along B1 (Figure 8b).
Vein
Venous control is done in two steps. The posterior branch (V2) of the central vein is dissected and clipped (Figure 9a). To approach V1, the lobe is retracted backward. The mediastinal pleura is incised posterior to the phrenic nerve. V1 is the uppermost branch of the superior pulmonary vein (Figure 9b). Its dissection is achieved by a combination of bipolar cautery and a gentle sweeping motion with an endopeanut.
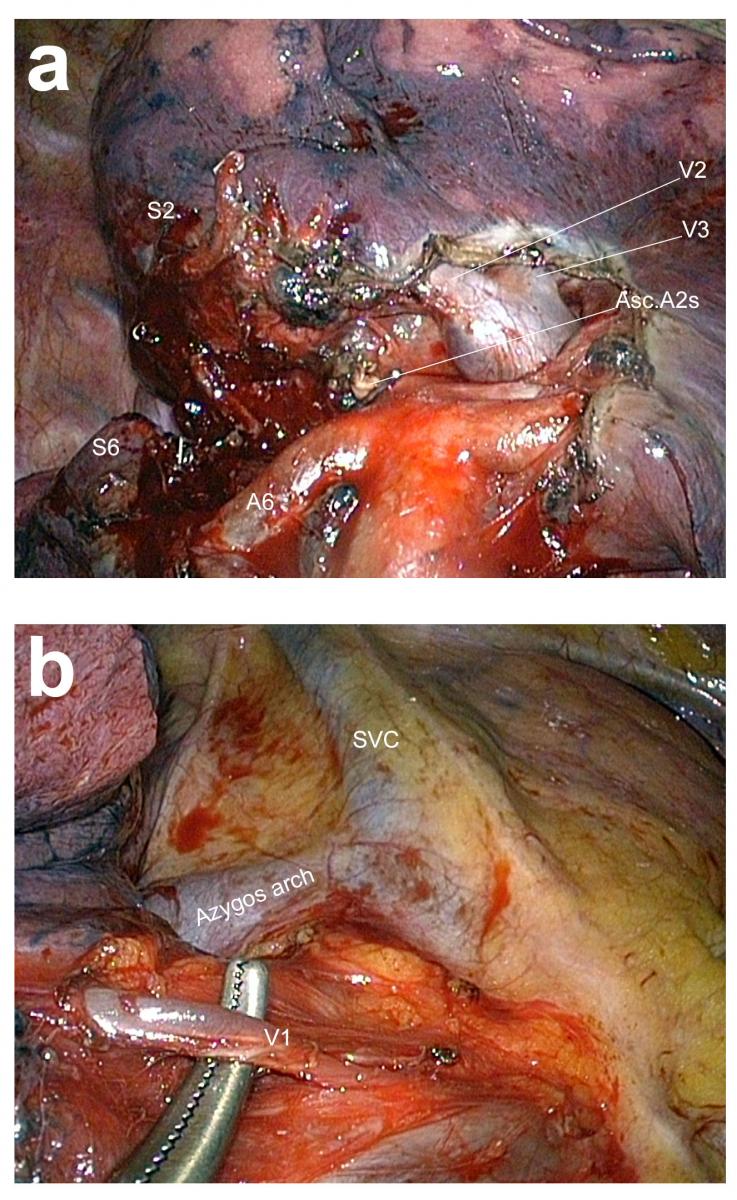
Figure 9. Control of segmental veins. a) Control of the posterior branch of the central vein (V2) in the fissure. b) Control of V1 in the mediastinum.
Division of the Parenchyma
Before stapling the intersegmental plane, one must check that the bronchial stump will not be caught within the stapler jaws. It must be dissected and forced back to be kept away (Video 6). The lobe can be lifted and a clamp is applied on the parenchyma. Low volume and low pressure reventilation help determe the intersegmental plane while the clamp is repositioned on the demarcation line between ventilated and nonventilated zones. Some force is applied on the clamp to crush and flatten the parenchyma. The 60 mm endostapler with a 4.8 mm cartridge is then applied (Video 7).
The first stapling is done in an anterior direction. The second one has a more anterior direction, making sure that the anterior subsegment of S1 (S1b) will be resected with the specimen (Figure 9). During positioning of the endostapler, the surgeon must confirm that no clip or staple will conflict with the creation of the staple line.
The same is true for the distal bronchial stump, which must be kept away from the stapler jaws (Figures 10 and 11). The specimen is placed into an endobag and retrieved in the usual fashion.
Preference card
- High-definition deflectable 10 mm thoracoscope
- Scope holder
- Endostapler with curved tip
- Clip applier
- Thoracoscopic instruments kit
- Blood deflecting trocar
- Vessel-sealing device
- Bipolar forceps
- Endopeanuts
- Specimen retrieval bag
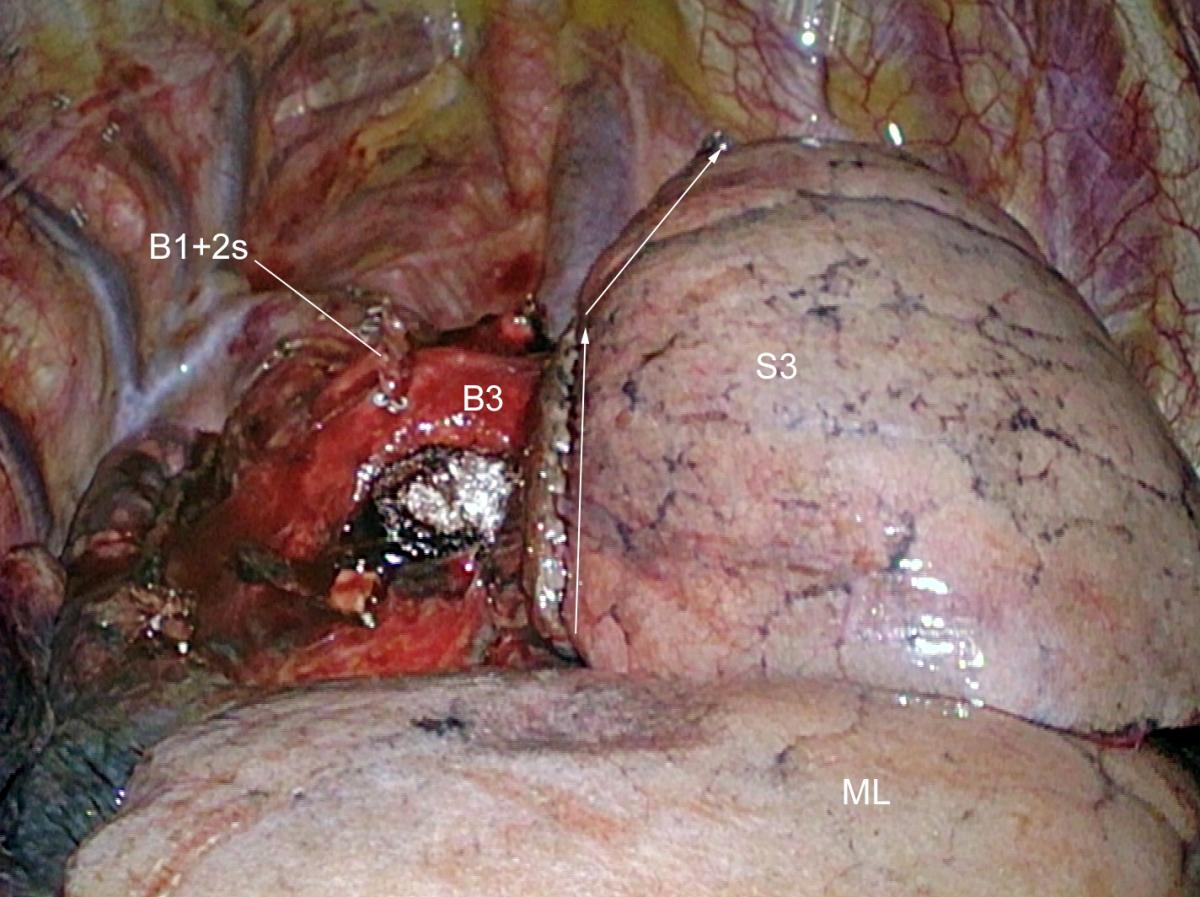
Figure 10. Final aspect after retrieval of segments 1 and 2. White arrows indicate the direction of stapling.
Pitfalls
- When there are two ascending A2, the most anterior one must not be mistaken for an anterior ascending branch (A3), which supplies the anterior segment.
- When present, the recurrent A2 can be a danger during dissection of B1 or B2.
- In about 10% of patients, the ascending A2 artery originates from the superior supply of the lower lobe or forms a common trunk with A6.
- A small vein draining S2 frequently crosses the posterior aspect of the ascending A2 and must be severed before dissecting this artery (Figure 2d).
- During a posterior approach, such as the technique described here, B2 can be mistaken for B1+2 if B1 and B2 are independent and if both bronchi are short (B1 will appear once B2 is stapled).
Results
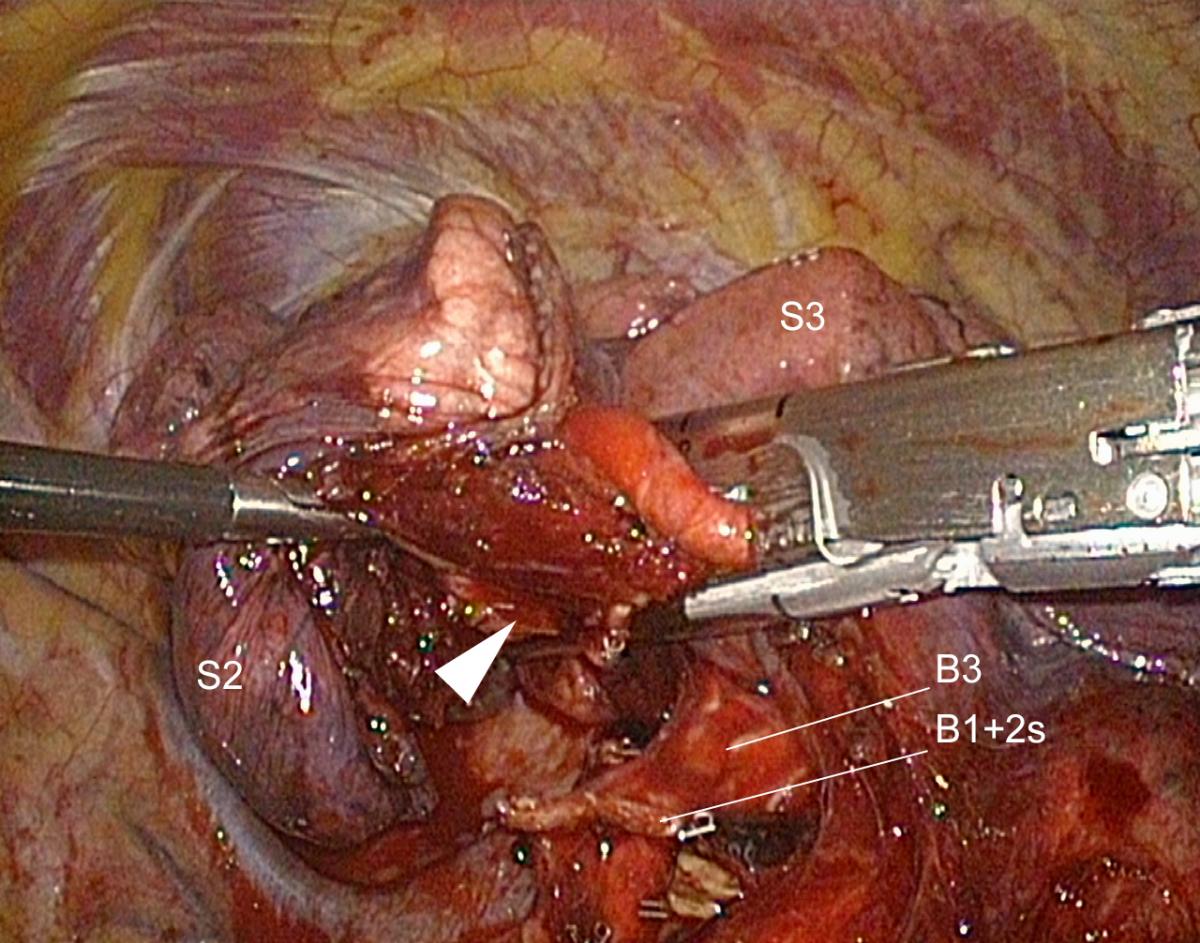
Figure 11. The white arrow indicates the bronchial stump that must be kept away from the staple line.
From January 2007 to June 2017, the authors performed 375 segmentectomies using a full thoracoscopic approach. Of these, 63 were a right S1+2, for suspicion of NSCLC (n = 47), metastasis (n = 5), or other condition (n = 11). In the group of patients operated on for a suspected NSCLC, the median larger diameter of the tumor was 18 mm (range: 8 - 25 mm).
There were 31 males and 33 females with a mean age of 64.2 ± 9.1. Their median body mass index was 24.1 ± 4.6.
Mean duration of the procedure, skin to skin, was 154 min (range: 77 - 407 min). Blood loss ranged from 0 - 1700 ml with a mean of 80 ml.
Two procedures were converted to an upper lobectomy, one for a vascular accident and one because of doubtful resection margins on frozen section. There was no conversion into thoracotomy in this group, although it was 3.9% in our total series of segmentectomies.
Median duration of chest drainage was three days (1 - 13 days), and median postoperative length-of-stay was five days (3 - 22 days).
One patient died from an acute respiratory distress syndrome (1.5%). There were 13 complications (15.8%): eight prolonged air leaks (none of which required reoperation), two pneumothorax, one pneumonia, one empyema, and one pulmonary embolism.
Comments
Despite their apparent complexity, S1+2 segmentectomies are easier than some sublobar resections, as the direction of the intersegmental plane is found more easily and does not pose many problems. The technique is similar to the one used for right upper lobectomy, but the dissection is just pushed one step further.
References
- Gossot D. Atlas of endoscopic major pulmonary resections. Paris: Springer-Verlag; 2010. 170 p.
- Gossot D, Ramos R, Brian E, Raynaud C, Girard P, Strauss C. A totally thoracoscopic approach for pulmonary anatomic segmentectomies. Interact Cardiovasc Thorac Surg. 2011;12(4):529-532.
- Nomori H, Okada M. Anatomical Segmentectomy for Lung Cancer. Tokyo: Springer-Verlag; 2012.
- Cao C, Chandrakumar D, Gupta S, Yan TD, Tian DH. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer. 2015;89(2):121-132.
- Aokage K, Saji H, Suzuki K, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg. 2017;65(5):267-272.
- Cao C, Gupta S, Chandrakumar D, Tian D, Black D, Yan T. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg. 2014;3(2):134-141.
- Cox M, Yang C, Speicher P, et al. The Role of Extent of Surgical Resection and Lymph Node Assessment for Clinical Stage I Pulmonary Lepidic Adenocarcinoma: An Analysis of 1991 Patients. J Thorac Oncol. 2017;12(4):689-696.
- Yang Q, Xie B, Hu M, Sun X, Huang X, Guo M. Thoracoscopic anatomic pulmonary segmentectomy: a 3-dimensional guided imaging system for lung operations. Interact Cardiovasc Thorac Surg. 2016;23(2):183-189.
- Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery. Eur J Cardiothorac Surg. 2014;46(6):e120-126.
- Kanzaki M, Kikkawa T, Shimizu T, et al. Presurgical planning using a three-dimensional pulmonary model of the actual anatomy of patient with primary lung cancer. Thorac Cardiovasc Surg. 2013;61(2):144-150.