ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
An Interesting Source of Hemoptysis
A 52-year-old man presented to the emergency department with complaints of hemoptysis for two days (1/2 to 1 cup of bright blood). At the time of admission, the patient reported a two-day history of fever (102o F) and chills, but denied weight loss or night sweats.
Patient Presentation
A 52-year-old man presented to the emergency department with complaints of hemoptysis for two days (1/2 to 1 cup of bright blood). At the time of admission, the patient reported a two-day history of fever (102o F) and chills, but denied weight loss or night sweats. He had a significant past medical history for an episode of hemoptysis in 1990. Chest x-ray at that time showed a left upper lobe (LUL) cavity with positive cultures for coccidioides immitis (BAL). In addition, he suffered a myocardial infarction (MI) in August 2002. The patient denied personal or family history of lung malignancy or tuberculosis. He had a 40 pack-year history of smoking, but quit soon after experiencing his MI in 2002. His current alcohol consumption is approximately two beers per day, decreased from 6-12 beers prior to his MI. The patient had traveled and lived in the southwest United States during the 1990’s.
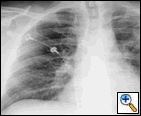
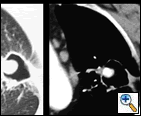
The patient was hemodynamically stable and his physical examination was unremarkable. His laboratory examination did not reveal any electrolyte abnormalities or anemia. An admission chest roentgenogram was obtained and revealed a LUL abnormality (Figure 1). Consequently, a computed tomogram (CT) was obtained and confirmed the LUL abnormality as a thick-walled 2 cm LUL cavity and also revealed the presence of a contrast enhanced, 7 mm mural, soft tissue nodule with the cavity (Figure 2). A high-resolution CT with three dimensional reconstruction demonstrated communication from the LUL nodule to vessels adjacent to the cavity wall (Figure 3).
Bronchoscopy was unremarkable for gross masses, but washings were positive for coccidioides immitis. Secondary to increasing hemoptysis, the patient underwent an emergent left upper lobectomy utilizing a standard posterolateral thoracotomy incision.
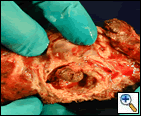
Gross inspection revealed a 4.0 x 3.0 x 1.5 cm cavity in the superior medial aspect of the LUL and represented an aneurysm from the pulmonary artery (Figure 4). Intraoperative frozen section of the LUL demonstrated a cavitary lesion surrounded by necrotizing granulomatous inflammation. Left upper lobe tissue subsequently grew coccidioides immitis. The patient had an uneventful post-operative course and remains well on six-month follow-up.
Comments
Solitary peripheral pulmonary artery aneurysms (PAAs) are a rare entity and have been sparingly reported [1-5]. Etiologic factors include trauma, infection, pulmonary hypertension, and congenital or acquired pulmonary vascular abnormalities. Although PAAs are often referred to as Rasmussen aneurysms, in the purest sense, a Rasmussen aneurysm is a complication of cavitary tuberculosis [2-3]. Hemoptysis ensues as compromise of the arterial wall integrity leading to pseudoaneurysm formation with vessel rupture and subsequent hemoptysis. In the current patient, reactivation of a latent coccidiodes immitis infection initially acquired over 12 years ago appears to have been the impetus for formation and rupture of the PAA. Although not the case in the current patient, mycotic PAAs generally occur in immune compromised hosts, intravenous drug abusers, or in those with endocarditis [5]. The etiologic development of mycotic aneurysms has been attributable to direct extension into the media of a focus of infection in the lung, by hematological dissemination via the bronchial arteries, or by invasion of the intima at a site of septic embolism [5]. Mortality rates have been reported as high as 50% in those with hemoptysis and a mycotic PAA. The two primary modalities of treatment are embolization or surgery (aneurysmectomy, wedge resection, or lobectomy) [1-5].
References
- Fukai I, Masaoka A, Yamakawa Y, et al. Rupture of congenital peripheral pulmonary aneurysm. Ann Thorac Surg 1995;59:528-30.
- Winer-Muram HT and Rubin SA. Thoracic complications of tuberculosis. J Thorac Imaging 1990;5:46-63.
- Kim HY, Song K-S, Goo JM, Lee JS, Lee KS, Lim T-H. Thoracic sequelae and complications of tuberculosis. RadioGraphics 2001;21:839-58.
- Nair KKS, Cobanoglu AM. Idiopathic main pulmonary artery aneurysm. Ann Thorac Surg 2001;71:1688-90.
- Benveniste O, Bruneel F, Bedos JP, et al. Ruptured mycotic pulmonary artery aneurysm: an unusual complication of right-sided endocarditis. Scan J Inf Dis 1998;30:626-9.