ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
AIRFIX®: Technical Features of the First Digital Airflow Measurement Device for Bedside Use
Introduction
Air leaks continue to be the most common complication after lung surgery, as well as an accepted morbidity. Minor leaks are generally innocuous and can be managed conservatively by chest tube drainage; but prolonged leaks, defined as those lasting more than 7 days, may require further interventions resulting in a delay of discharge and thus causing additional costs [1-3]. Currently there is little consensus on the management of prolonged air leaks. Published treatment modalities, including changing the amount of suction, placement of additional chest tubes, chemical pleurodesis, or even reoperation, are usually based on semi-quantitative measurements or on only approximate assessments of air loss through drains by mere inspection [4-7]. Because of the lack of objective data on air loss across the lung surface, "tentative treatments" often precede the adequate one. We now describe a device that enables quantitative assessment of air loss as a means to improve algorithms for managing air leaks after pulmonary surgery.
General technical description
The measuring device (AIRFIX®; TEUP's Ltd., Deutschlandsberg, Austria) (Figure 1) is based upon a "mass airflow" sensor with a specially designed software package. An embedded data acquisition system performs basic gain and offset calculations and continuously sends measurement values over a serial data link to a hand held processor, from which data can be transmitted to any desktop PC for storage and/or printout. Only the net flux in one direction (from the drainage tube into the device) is recorded as air leak. The amount of air moving into the opposite direction (shift of intrapleural dead space, e.g. after a lobectomy) is subtracted both from the leakage per breath and from the leakage per minute. A disposable catchment tank for fluids is installed proximal to the inflow-port of the system, preventing spilling of blood or serous fluids from the chest tube into the measuring device.
For clinical use the inflow port of the AIRFIX-device is connected in line with the chest tube (Figure 2). The drainage system is plugged to the outflow-port. The connection at the ports is provided by disposable screw plugs. If suction based drainage systems are connected to fittings set at high negative pressure levels, profuse formation of bubbles occurs in the system, which may cause measuring artefacts due to the high sensitivity of the device. In such cases, the degree of negative pressure at the fitting has to be reduced during measurements or the outflow port has to be temporarily connected to a Heimlich-valve in order to establish optimal measuring conditions. Measurement data are recorded at a rate of 10 single measurements per second. In addition, the plot contains markers indicating different breathing manoeuvres. Following each measurement the data acquired can be printed out in an airflow report (Figure 3) to be stored with the documents of the respective patient.
Technical properties
- Capabilities of the Measuring System
The loss/time/volume (LTV) [ml/min] is measured. These data are based on loss/breath [ml/breath] and respiratory rate [breaths /minute]. - Measuring Range
0 to 5000 ml/min - Accuracy
In order to definitely proof an air leak, an accuracy of +-10 ml/min has to be provided. This results in an accuracy range of +- 5% of the measured value. The device should be kept horizontal during the measuring procedure - Bidirectionality
Since it is possible that a rapid reversal in flow direction occurs (e.g. in the inspiration phase), we use a sensor which can measure bidirectionally. - Sterilizability
In order to guarantee safe use for patients and medical personnel, the measuring device is gas sterilizable (ETO-gas). -
Functioning Principle of the Sensor
A mass flow rate sensor is used. The measuring pick-up is based on a silicon chip composed of a thermally insulated thin-film bridge structure. Both a heating element and temperature sensors are found on the chip. In order to recognise the flow direction, the sensor also operates as a differential anemometer. To provide this, two resistance elements are positioned on both sides of a central heating element. The resistance elements are implemented as thin-film resistors. The small dimensions of the resistance elements and the thermal insulation achieved by the mechanical structure in the form of a bridge guarantee high sensitivity and fast response times. - Size
Length x breadth x height: 125 x 105 x 65 [mm]; Weight: ~ 250g
Clinical data
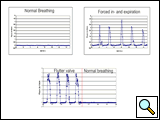
In a trial of 204 patients [8], we demonstrated the efficacy of this novel tool for evaluating air leaks following pulmonary resections. The intent of this project was to develop a safe, reproducible, and clinically applicable system for bed-side use. The device was designed to be connected in-line with the chest tube. In our patients suction drainage systems were used. The degree of suction during the measurements followed a standard protocol for evaluating the device, which included -20 cm H2O, -12 cm H2O, and Heimlich valve on the first postoperative day, and -12 cm H2O and Heimlich valve during the following days. At each suction level the patients were asked to breathe normally, to take deep breaths, to cough, and to breathe out against the resistance of a flutter valve (Figure 4). The leakage per expiration, as well as the leakage per minute, were displayed as standard parameters and recorded on the computer.
We found that a statistical comparison of the data obtained from measurements of identical suction levels (i.e. –20 mm Hg or -12 mm Hg or Heimlich valve) but during different breathing manoeuvres (i.e. normal breathing, deep breath, cough and flutter valve) showed significant differences. The validation of the AIR-FIX system by comparing the leakage with the one measured by intraoperative spirometry showed almost identical values (+/-0.5 ml/breath). A total of 7296 measurements were performed (mean: 36 / patient; range: 28 to 92). A measurement series (8 or 12 measurements, respectively) took 10 to 15 minutes. Depending on the suction level and the type of breathing manoeuvre, air leaks within the range of 0.25 ml/breath to 45 ml/breath, or 5 to 900 ml/minute, were documented.
We believe that precise quantification of the air leak might help to develop treatment algorithms and to define the correct moment for drain removal, thereby reducing morbidity and treatment costs.
Conclusion
The AIRFIX® device fulfils many requirements for the introduction of an evidence-based algorithm for the evaluation and treatment of air-leaks by providing full quantification facilities, and thus more reliable decision making, on chest tube adjustment and removal.
References
- Varela G, Jimenez MF, Novoa N, Aranda JL. Estimating hospital costs
attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac
Surg 2005;27:329-33. - Abolhoda A, Liu D, Brooks A, Burt M. Prolonged air leak following radical
upper lobectomy: an analysis of incidence and possible risk factors. Chest
1998;113:1507-10. - Wright CD, Wain JC, Grillo HC, Moncure AC, Macaluso SM, Mathisen DJ.
Pulmonary lobectomy patient care pathway: a model to control cost and
maintain quality. Ann Thorac Surg 1997;64:299-302. - Cerfolio RJ, Tummala RP, Holman WL, Zorn GL, Kirklin JK, McGiffin DC,
Naftel DC, Pacifico AD. A prospective algorithm for the management of air
leaks after pulmonary resection. Ann Thorac Surg 1998;66:1726-31. - Cerfolio RJ, Bass C, Katholi CR. Prospective randomized trial compares
suction versus water seal for air leaks. Ann Thorac Surg 2001;71:1613-7. - Cerfolio RJ, Sale Bass C, Harrison Pask A, Katholi CR. Predictors and
treatment of persistent air leaks. Ann Thorac Surg 2002;73:1727-31. - Ohri SK, Oswal SK, Townsend ER, Fountain SW. Early and late outcome
after diagnostic thoracoscopy and talc pleurodesis. Ann Thorac Surg
1992;53:1038-41. - Anegg U, Lindenmann J, Matzi V, Mujkic D, Maier A, Fritz L, Smolle-Jüttner
FM. AIRFIX: the first digital postoperative chest tube airflowmetry - a novel
method to quantify air leakage after lung resection. Eur J Cardiothorac Surg
2006;29:867-72.