ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Radiofrequency Ablation of Lung Tumors
Radio frequency ablation (RFA) of lung tumors is a relatively new procedure allowing local treatment with minimal parenchymal damage. In fact, this technique is able to induce coagulative necrosis in a limited pulmonary area.
Radio frequency ablation (RFA) of lung tumors is a relatively new procedure allowing local treatment with minimal parenchymal damage. In fact, this technique is able to induce coagulative necrosis in a limited pulmonary area [1]. This emerging technology is used to achieve local treatment of primary and secondary lung tumors in patients with poor clinical status or technical contraindications to surgical resection [2,3]. Pre-operative patient assessment is the same as for major pulmonary resection. The current election criteria include: maximum diameter of the tumor less than or equal to 5 cm; lesions located more than 1 cm from major blood vessels or airways; and patients with a platelet count of more than 50,000.

Figure 1. The generator has multiple temperature displays as well as impedance and power monitoring. A personal computer with dedicated software is connected to record and graphically represent all data.
Device
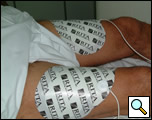
We describe hereby one of the systems currently in use to produce monopolar radiofrequency energy. This is an automatic apparatus with a maximum power output of 150 W, operating at 460 Hz (Model 1500, RITA Medical System, Mountain View, CA). It has multiple temperature displays as well as impedance and power monitoring, and software is available to record and graphically represent all data on a personal computer (Figure 1). The energy is transferred to the tissue by means of a multi-tined expandable array (StarBrust XL, RITA Medical system, Mountain View, CA). It consists of a 15-gauge needle cannula with nine deployable electrodes which open flower-like up to 5 cm (Video 1). Five electrodes are equipped with thermocouples that allow continuous measurement of the temperature inside the tissue. Two grounding pads are applied to each shaved leg to ground the current and to reduce risks of skin thermal injury (Figure 2). Once the system is switched on, the physician sets the parameters for the ablation procedure: the mode of operation, the target temperature, the ablation time, and the maximum power delivery level, all which can be modified at any time during the procedure.
Percutaneous thermal tissue ablation is performed with a needle electrode inserted into the lesion under imaging guidance; the tip has a non–insulated portion that generates medium frequency electromagnetic waves of 400 – 500 kHz; the patient is transformed into an electrical circuit with adhesive grounding pads on the thighs or back. The insertion technique is similar to a routine percutaneous biopsy. The mechanism of tissue heating for radiofrequency ablation is frictional or resistive energy loss caused by the motion generated by the ionic current. Alternating radiofrequency current agitates ions in the tissue surrounding the needle creating frictional heat, achieving a target temperature of about 90° C. This denatures and destroys tissue at predictable temperatures, in a relatively predictable volume; thus, the procedure results in tissue heating, coagulation necrosis and irreversible cell death. Recent technologic improvements allow the creation of thermal necrosis volumes up to 5 cm in diameter with a single percutaneous insertion of new multiple electrode devices, thus enabling successful ablation in a single treatment session.
Procedure
The procedure is performed with the patients under conscious sedation (Ketorolac 0.5-0.8 mg/kg, propofol 1-2 mg/kg/h, and remifentanil 0.1mg/kg/min) and local anesthesia (subcutaneous 1% xylocaine). Vital signs are continuously non-invasively monitored. CT guidance is employed in most of the cases; it is usually enhanced by the administration of contrast material before and after coagulation to obtain information about the real effectiveness of the procedure (Figure 3). In a selected group of patients with the tumour in contact with the thoracic wall, it is possible to work under ultrasound guidance (Video 2). The needle-electrode is inserted through an intercostal space after administration of local anaesthesia (Video 3). The correct placement is confirmed by CT (Figure 4) or ultrasound (Video 4) before applying the radiofrequency energy. The target temperature of ablation is 90°C. It is maintained for a time ranging from 15 to 27 minutes according to the size of the tumor; this variable also determines the gradual deployment of the electrodes, starting from 2 cm and then 1 cm for each step. When technically feasible, the ablation zone should include the whole lesion and one cm of the surrounding lung parenchyma.
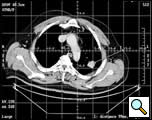
Figure 3. By CT guidance it is possible to establish the site, depth, and angle for insertion of the needle electrode.
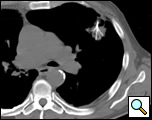
Figure 4. A CT scan confirmed the correct placement of the needle with the deployment of the electrodes into the tumor.
After RFA, the ablation zone is generally visible at CT scan as a ground glass opacity surrounding the target lesion (Figure 5). After the procedure the patient is transferred to the recovery room for observation. Twenty-four hours later, after performing a chest x-ray to exclude the occurrence of any complication (e.g. pneumothorax), the patient is discharged.
Radiological follow-up includes contrast-enhanced CT at 1, 3 and 6 months and then at 6-months intervals (Figure 6). Due to a frequent increase in density of the lesion immediately after RFA, usually related to the presence of an ablation zone larger that the lesion itself, at the 1st and 3rd month follow-up the main criteria for complete response is reduction of the contrast material enhancement, when compared to the measures taken before the procedure. However, a density higher than 25 Hounsfield Unit (HU) is considered indicative of persistence or relapse of the disease. The diameter of the lesion becomes the main criterion after the 6th month, in accordance with the RECIST criteria [4]. An increase of the size of the lesion is considered to be persistent or relapsing disease. However, both criteria (size and enhancement) should be evaluated and integrated on a case by case basis to determine whether progression occurs. If available, PET and CT-PET scans are useful in follow-up of these patients.
Results
The first percutaneous RFA of lung lesions is reported by Dupuy in 2000 [5]. He successfully treated three patients with palliative intent. Since then, a number of reports have described the feasibility and safety of the procedure. In particular, an international survey with almost 500 RFA of lung tumors reported by Steinke and colleagues in 2004 stressed the low invasiveness approach of this technique, the negligible mortality, low morbidity, short hospital stay, and quality of life improvement [6].
Most of the reports address short term results with encouraging data. Herrera [3] treated a mixed cohort of 18 patients (5 via thoracotomy and 13 percutaneously) with primary and metastatic lung tumors. They utilized a needle electrode with multiple tines deployable into the tumor for 2 or 4 cm. After a mean follow-up of 6 months they reported a 55% complete or partial response rate and a 17% stable disease rate in lesions with a mean diameter of 5.3 cm. The response rates seemed to be better for smaller lesions (66% for lesions smaller than 5 cm vs. 33% for lesions larger than 5 cm). Another important issue which arose in that study was the contraindication to treat central lesions due to the risk of fatal complications, as happened in one case in that series. More recently, Fernando and colleagues from the same institute reported an update of their experience in patients with primary NSCLC [7]. At a mean follow-up period of 14 months they reported 63% of the patients having a complete response. Akebosi et al, in a series of 54 primary and secondary lung tumors reported a complete necrosis rate of 59%, with a better response for smaller lesions [8]. They distinguished lesions smaller than or equal to 3 cm from lesions greater than 3 cm, and found a statistically significant difference in the complete response rate between the two groups (69% vs 39%; p<0.05). These findings have been confirmed by Lee and colleagues [9], who reported a complete necrosis rate of 38% at a mean follow-up of 12.5 months with a statistically significant difference for smaller lesions than 3 cm (100% complete necrosis rate) compared to those measuring 3 – 5 cm (38%; p<0.05), and those >5 cm (only 8% complete necrosis rate). They utilized either a single electrode or multiple deployable electrode needles according to the size of the lesion; in most of the patients several needle insertions were performed. Steinke et al. [10] reported their experience with RFA of lung metastases from colorectal cancer. In their series, lesions ranging from 0.3 to 4.2 cm disappeared, or were reduced in size or stable at CT after 12 months of follow up in 65% of the cases.
We recently reported our experience with medium term results [11]. Our data confirm all the previous reports, with an overall radiological complete response rate (CRR) of 61.9% (39/63 lesions) at a mean follow-up period of 23.7 months (median 24, range of 6–50). The results appeared better for lesions smaller than 3 cm (CRR 69.7% vs. 50%) and for metastases from extra-thoracic malignancies (CRR 70.8% vs. 56.4%), even though they didn’t reach statistical significance. In our study, pulmonary function tests performed before and after RFA at several time points showed a slight reduction of FVC and FEV1 at the end of the first month (not statistically significant), but returned to pre–treatment values after three months.
It is important to stress that RFA is devoted to local treatment of lesions, and this is certainly a limitation of this procedure. When compared to surgical resection it has a higher incidence of local and distant recurrence. For this reason, this procedure should only be intended as a compromise in treating patients who are at high risk for standard therapy.
References
- Ambrogi MC, Fontanini G, Lencioni R, et al. A preliminary study on thermal ablation of lung tumour. Contemporary Oncology 2004;8:373-79.
- Steinke K, King J, Glenn D, Morris DL. Radiologic appearance and complications of percutaneous computed tomography-guided radiofrequency-ablated pulmonary metastases from colorectal carcinoma. J Comput Assist Tomogr 2003;27:750-7.
- Herrera LJ, Fernando HC, Perry Y, et al. Radiofrequency ablation of pulmonary malignant tumors in non surgical candidates. J Thorac Cardiovasc Surg 2003;125:929-37.
- Padhani AR, Ollivier L. The RECIST (Response Evaluation Criteria in Solid Tumors) criteria: implication for diagnostic radiologists. Br J Radiol 2001;74:983-6.
- Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneus radiofrequency ablation of malignancies in the lung. Am J Roentgen 2000;174:57-59.
- Steinke K, Sewell PE, Dupuy D, et al. Pulmonary radiofrequency ablation—an international study survey. Anticancer Res 2004;24:339-43.
- Fernando HC, De Hoyos A, Landreneau RJ, et al. Radiofrequency ablation for the treatment of non-small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg 2005;129:639-44.
- Akeboshi M, Yamakado K, Nakatsuka A, et al. Percutaneous radiofrequency ablation of lung neoplasms: initial therapeutic response. J Vasc Interv Radiol 2004;15:463-70.
- Lee JM, Jin GY, Goldberg SN, et al. Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastasis: preliminary results. Radiology 2004;230:125-34.
- Steinke K, Glenn D, King J, et al. Percutaneous imagiong-guided radiofrequency ablation in patients with colorectal pulmonary metastases: 1-year follow-up. Ann Surg Oncol 2004;11:207-12.
- Ambrogi MC, Lucchi M, Dini P, et al. Percutaneous radiofrequency ablation of lung tumours: results in the mid-term. Eur J Cardiothorac Surg 2006;30:177-83.