ALERT!
This site is not optimized for Internet Explorer 8 (or older).
Please upgrade to a newer version of Internet Explorer or use an alternate browser such as Chrome or Firefox.
Surgical Management Of Epiphrenic Diverticula
Patient Selection
Epiphrenic diverticuli are acquired mucosal outpouchings protruding through the muscular wall of the distal 10 cm of the esophagus [1]. Most are associated with an underlying esophageal motility disorder. The functional obstruction caused by the motility disorder and the resultant increased intraesophageal pressure are thought to provide the stimulus for the genesis of these pulsion diverticula.
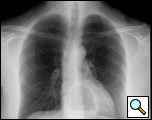
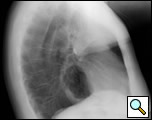
The clinical manifestations are unpredictable and are not influenced by the size of the diverticulum but rather correlate with the underlying motility disturbance. Dysphagia and regurgitation are the predominant symptoms observed. Respiratory complaints, when present, indicate aspiration. Most esophageal diverticuli are found incidentally. Chest radiography may be normal or may reveal a mediastinal density or air-fluid level (Figure 1). This feature may be misdiagnosed as a hiatus hernia. The diagnosis of an epiphrenic diverticulum is usually made on an upper gastrointestinal series (Figure 2). Oblique views can define the size, location, number and shape of diverticula. Contrast studies, however, are unreliable for evaluating motility patterns and thus esophageal manometry should be performed preoperatively. Correct placement of the catheter can be difficult and may be assisted with endoscopic guidance. Esophageal endoscopy is recommended to exclude other etiologies for dysphagia. This maneuver can be difficult and should be performed by an experienced endoscopist.
Given the rarity of epiphrenic diverticuli, there is considerable debate over what constitutes standard therapy. Patients who exhibit moderate to severe esophageal symptoms should undergo operative treatment. A proportion of patients is asymptomatic or has sufficiently minimal symptoms that they don’t warrant treatment. These patients may be followed with little risk of progression, although the management of this cohort is a matter of debate [2,3].
Operative Steps
Open transthoracic surgery represents the traditional approach for the treatment of epiphrenic diverticuli and is our preference. However, it should be noted that minimally invasive techniques, including thoracoscopic and laparoscopic transhiatal methods have been reported with success [4-8]. Although technical feasibility has been demonstrated with minimally invasive approaches, some authors have reported significant morbidity and long- term outcome data is limited [8]. As pointed out by Allen, these minimally invasive procedures are attractive but further development is needed before they can be recommended as acceptable therapy [3]. For the purposes of this review, we will describe only the open transthoracic approach.
The patient is positioned on the operating table in the right lateral decubitus position with generous padding of all bony prominences. Surgery is best conducted under single lung ventilation. This can be achieved with a double lumen endotracheal tube or bronchial blocker. There should be nothing placed in the esophagus prior to the operation. Attempts at passing a nasogastric drain or esophageal stethoscope can lead to perforation and should be avoided.
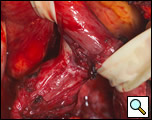
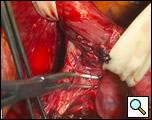
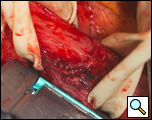
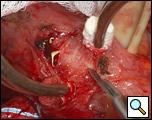
The surgical approach is a left posterolateral thoracotomy, entering the chest over the bed of the unresected 7th or 8th rib. On entering the thoracic cavity, the pulmonary ligament is divided and the lung is packed out of the way superiorly. The epiphrenic diverticulum is often found at the level of the inferior pulmonary vein. The pleura over the esophagus is divided allowing the esophagus to be gently mobilized. Penrose drains are used to encircle the esophagus superior and inferior to the diverticulum to facilitate the dissection (Figure 3). The diverticulum often arises to the right side necessitating rotation of the esophagus to clearly visualize the neck of the diverticulum. The diverticulum and its base are carefully dissected from the adjacent esophagus (Figure 4). Both vagus nerves are clearly identified and preserved during this maneuver. Once the diverticulum base is defined, a 40 Fr bougie is introduced across the gastroesophageal junction. This is performed under direct vision with the surgeon closely monitoring the safe passage of the bougie.
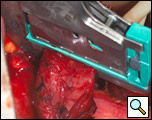
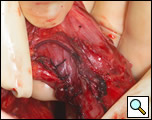
The diverticulum is then amputated at its base with a TA-60, 4.8 stapling device (Figure 5). The staple line is kept parallel to the long axis of the esophagus to avoid tension and decrease the risk of leak (Figure 6). The diverticulum is amputated sharply with a scalpel (Figure 7) and the staple line reinforced by approximating the muscularis with interrupted 3-0 silk sutures (Figure 8).
Our preference is to perform a diverticulectomy for all patients’ epiphrenic diverticula. It should be noted that a selective approach to these diverticula has also been advocated. This approach would include performing a diverticulectomy only in situations where the diverticulum is known to cause symptoms. It is not clear if this approach reduces the incidence of suture line leak or stricture formation. In our experience, routine diverticulectomy adds minimal additional morbidity. Additionally, it can be argued that large diverticula (> 4 cm) should be resected, even if asymptomatic, due to the inherent risk of aspiration.
The underlying abnormality of epiphrenic diverticulum is an esophageal motility disorder, thus this condition should be addressed at the time of surgery with a long extramucosal esophagomyotomy. The myotomy will prevent suture-line leak at the diverticulectomy site by relieving distal obstruction, in addition to decreasing diverticular recurrence and relieve symptoms of the underlying motility condition.
The myotomy is performed opposite the site of the diverticulectomy and should be carried onto the stomach (1). The myotomy is created using a scalpel to carefully divide the muscle layers (Figure 9). The divided edge of muscularis propria is gently mobilized laterally for half of the circumference of the esophagus (Figure 10). Electrocautery is rarely necessary and should be avoided in trying to attain hemostasis because of the risk of thermal injury to the exposed mucosa and subsequent delayed perforation.
 |
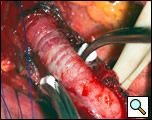 |
| Figure 9: Creation of the esophagomyotomy with a scalpel. | Figure 10: Mobilization of the divided edges of the myotomy. |
The length of the esophageal myotomy is tailored to the underlying motility disorder. The cephalad extent of the myotomy should be through all regions of the esophagus documented to have abnormal motility. If the motility is normal, the myotomy is carried to a level above the diverticulum, which is typically between the inferior pulmonary vein and the aortic arch [1]. If the patient had achalasia, a limited myotomy involving the distal esophagus, the lower esophageal sphincter and extending onto the gastric cardia will suffice, whereas in patients with diffuse esophageal spasm or non-specific motility disorder, the myotomy should be extended superiorly to the level of the aortic arch. The myotomy should include the lower esophageal sphincter if manometry documents a hypertensive lower esophageal sphincter and if the manometry is normal, a shorter myotomy is performed, sparing the lower esophageal sphincter.
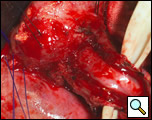
There is considerable debate over the addition of an antireflux procedure after the completion of the myotomy [3,6]. Although there is no consensus regarding the performance of a fundoplication, a partial nonobstructing antireflux procedure such as the modified Belsey Mark IV is preferable to a complete wrap [1,6]. One advantage to an antireflux procedure is the prevention of acid reflux following the disruption of the gastroesophageal junction, which may occur if the myotomy is extended onto the gastric cardia. Prior to mobilization of the stomach for the wrap, it is advantageous to first place the cruroplasty sutures. This is done with nonabsorbable suture such as #1 polypropylene in a figure of eight configuration approximating the crura. The narrowed hiatus should allow the easy passage of no more than two fingers across the crural opening. Making the hiatal opening too narrow will result in postoperative dysphagia and leaving the opening too large may result in herniation. Following mobilization of the fundus into the thoracic cavity, two 2-0 silk sutures are placed in a horizontal mattress fashion 2 cm from the gastroesophageal junction on either side of the myotomy approximating the gastric fundus and the muscularis of the esophagus (Figure 11). Once these sutures are tied, a second identical row of two 2-0 silk sutures are placed 4 cm from the original gastroesophageal junction. These latter two sutures are also passed through the diaphragm reducing the fundoplication under the hiatus and securing the wrap to the underside of the diaphragm (Figure 12). The crural sutures are tied snugly, but not so as to create obstruction at the hiatus. Once hemostasis is achieved, a single pleural drain is left into the chest cavity and the incision is closed in layers.
 |
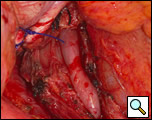 |
| Figure 11: Second row of silk sutures for Belsey partial fundoplication. | Figure 12: Completed Belsey partial fundoplication. |
Patients are not allowed to take oral feeding until a contrast esophagram is performed 24 to 48 hours postoperatively to exclude an unsuspected esophageal perforation. If the contrast study is normal, a clear liquid diet is initiated. The pleural tube is removed the following day if the drainage is less than 200-300 cc per day. Patients are discharged on a liquid diet for 2 weeks and are advanced progressively to solid food.
Preference Card
- Double lumen endotracheal tube
- TA-60 4.8 mm stapler
- 40 Fr bougie
- Kittner dissectors
- Penrose drain, ¼ inch
- #15 blade on a long handle
Tips & Pitfalls
- Complete collapse of the ipsilateral lung under single lung ventilation is essential for a clear visualization of the esophagus and diverticulum.
- Carefully dissect the neck of the diverticulum down to the level of the submucosa.
- Place the diverticulectomy staple line along the long axis of the esophagus and not perpendicular, which can increase the risk of esophageal leak.
- Delicately dissect the gastroesophageal junction and distal esophagus, avoiding injury to the vagus nerves.
- The esophageal myotomy should be tailored to the specific motility disorder if possible.
- Mucosal breaches are repaired with 5-0 polypropylene suture. Muscle reinforcement may not be possible.
Results
The optimal treatment of epiphrenic diverticula remains controversial. The Mayo Clinic review of 112 previously untreated patients with epiphrenic diverticula identified 71 who had absent or minimal symptoms who were successfully followed without symptom progression [1]. Of the 41 patients with symptoms, 33 patients underwent surgical repair. The most common procedure was diverticulectomy and esophagomyotomy. A Belsey antireflux procedure was the most common fundoplication performed. The reported complication rate was 33 % and the operative mortality was 9 %, but this decreased in recent years [2]. In another review, Hudspeth reviewed a 15-year experience of nine patients who underwent correction through thoracotomy without any operative mortality [9]. In the largest minimally invasive cohort of patients, complications occurred in 45% of patients with a 20 % leak rate and a mortality of 5%. Long-term results in the Mayo Clinic series are excellent to good in 76 %, fair in 17 % and poor in 7 % [2]. These results are comparable to those obtained through minimally invasive approaches [8]. Poor surgical results are often attributed to inadequate myotomy.
References
- Deschamps C and Trastek VF. Esophageal diverticula. In: Shields TW, LoCicero J, Ponn RB, ed. General Thoracic Surgery, fifth edition. Lippincott Williams & Wilkins, 2000:1839-1849.
- Benacci JC, Deschamps C, Trastek VF, Allen MS, Daly RC, Pairolero PC. Epiphrenic diverticulum: Results of surgical treatment. Ann Thorac Surg 1993;55:1109-14.
- Allen MS. Treatment of epiphrenic diverticula. Semin Thorac Cardiovasc Surg 1999;11:358-62.
- Rosati R, Fumagalli U, Bona S, Zago M, Celotti S, Bisagni P, Peracchia A. Laparoscopic treatment of epiphrenic diverticula. J Laparoendosc Adv Surg Tech 2001;11:371-5.
- Thomas ML, Anthony AA, Fosh BG, Finch JG, Maddern GJ. Oesophageal diverticula. Br J Surg 2001;88:629-42.
- Van der Peet DL, Klinkenberg-Knol EC, Berends FJ, Cuesta MA. Epiphrenic diverticula: minimal invasive approach and repair in five patients. Dis Esophagus 2001;14:60-62.
- Tescado P, Fisichella PM, Way LW, Patti MG. Cause and treatment of epiphrenic diverticula. Am J Surg 2005;190:891-4.
- Fernando HC, Luketich JD, Samphire J, Alvelo-Rivera M, Christie NA, Buenaventura PO, Landreneau RJ. Minimally Invasive Operation for Esophageal Diverticula. Ann Thor Surg 2005;80:2076-81.
- Hudspeth DA, Thorne MT, Conroy R, Pennell TC. Management of epiphrenic esophageal diverticula. A fifteen-year experience. Am Surg 1993;59:40-42.